User login
A Review of Online Search Tools to Identify Funded Dermatology Away Rotations for Underrepresented Medical Students
A Review of Online Search Tools to Identify Funded Dermatology Away Rotations for Underrepresented Medical Students
Most medical students applying to dermatology residency programs in the United States will participate in an away rotation at an outside institution. Prior to COVID-19–related restrictions, 86.7% of dermatology applicants from the class of 2020 reported completing one or more away rotations for their application cycle.1,2 This requirement can be considerably costly, especially since most programs do not offer financial support for travel, living expenses, or housing during these visiting experiences.3 Underrepresented in medicine (URiM) students may be particularly disadvantaged with regard to the financial obligations that come with away rotations.4,5 Visiting scholarships for URiM students can mitigate these challenges, creating opportunities for increasing diversity in dermatology. When medical students begin the residency application process, the Visiting Student Learning Opportunities (VSLO) program of the Association of American Medical Colleges (AAMC) is the most widely used third-party service for submitting applications. For many URiM students, an unforeseen challenge when applying to dermatology residency programs is the lack of an easily accessible and up-to-date search tool to find programs that offer funding, resulting in more time spent searching and thereby complicating the application process. The VSLO released the Visiting Scholars Resources Database, a search tool that aims to compile opportunities for additional support—academic professional, and/or financial—to address this issue. Additionally, the Funded Away Rotations for Minority Medical Students (FARMS) database is an independent directory of programs that offer stipends to URiM students. In this study, we evaluated the efficacy of the VLSO’s Visiting Scholars Resources Database search tool and the FARMS database in identifying funded dermatology rotations for URiM students.
Overview of Online Search Tools
We used the AAMC’s Electronic Residency Application Service Directory to identify 141 programs offering dermatology residency positions. We then conducted a Google search using each program name with the phrase underrepresented in medicine dermatology away rotation to identify any opportunities noted in the Google results offering scholarship funding for URiM students. If there were no Google results for a webpage discussing URiM away rotation opportunities for a certain program, the individual program’s website search box was queried using the terms URiM, scholarship, and funding. If there were no relevant results, the webpages associated with the dermatology department, away rotations, and diversity and inclusion on the respective institution’s website were reviewed to confirm no indication of funded URiM opportunities. Of the 141 dermatology programs we evaluated, we identified 56 (39.7%) that offered funded away rotations for URiM students.
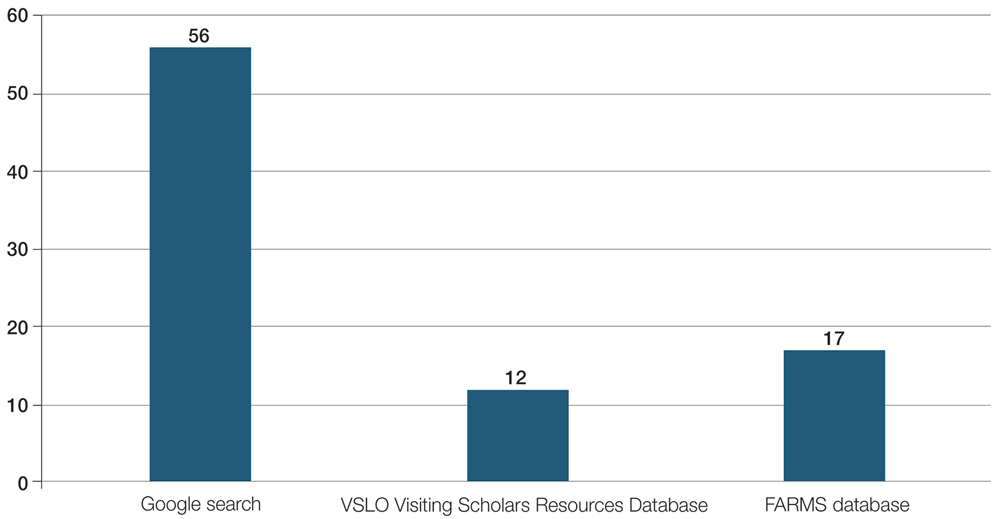
For comparison, we conducted a search of the VSLO’s Visiting Scholars Resources Database to identify programs that listed dermatology, all (specialties), or any (specialties) under the Specialty column that also had a financial resource for URiM students. Our search of the VSLO database yielded only 12 (21.4%) of the 56 funded away rotations we identified via our initial Google and program website search. Program listings tagged for dermatology also were retrieved from the FARMS database, of which only 17 (30.4%) of the 56 funded away rotations we previously identified were included. All queries were performed from October 24 to October 26, 2024 (Figure).
Comment
The 2023-2024 AAMC Report on Residents indicated that 54.9% (800/1455) of active US dermatology medical residents identified as White, 27.5% (400/1455) identified as Asian, 8.9% (129/1455) identified as Hispanic, and 8.7% (126/1455) identified as Black or African American.6 By comparison, 19.5% of the general US population identifies as Hispanic and 13.7% identifies as Black.7 Within the field of dermatology, the proportion of Black dermatology academic faculty in the US is estimated to comprise only 18.7% of all active Black dermatologists.8,9 With a growing population of minority US citizens, the dermatology workforce is lagging in representation across all minority populations, especially when it comes to Hispanic and Black individuals. To increase the diversity of the US dermatology workforce, residency programs must prioritize recruitment of URiM students and support their retention as future faculty.
Reports in the literature suggest that clinical grades, US Medical Licensing Examination scores, letters of recommendation/ networking, and the risk of not matching are among the primary concerns that URiM students face as potential barriers to applying for dermatology residency.4 Meanwhile, dermatology program directors ranked diversity characteristics, perceived interest in the program, personal prior knowledge of an applicant, and audition rotation in their department as important considerations for interviewing applicants.10 As a result, URiM students may have the diverse characteristics that program directors are looking for, but obtaining away rotations and establishing mentors at other institutions may be challenging due to the burden of accruing additional costs for visiting rotations.2,10,11 Other reports have indicated that expanding funded dermatology visiting rotations and promoting national programs such as the American Academy of Dermatology Diversity Mentorship Program (https://www.aad.org/member/career/awards/diversity) or the Skin of Color Society Observership Grant (https://skinofcolorsociety.org/what-we-do/mentorship/observership-grant) can be alternative routes for mentorship and networking.3
Our review demonstrated that, of the 141 dermatology residency programs we identified, only around 40% offer funded rotations for URiM students; however, the current databases that applicants use to find these opportunities do not adequately present the number of available options. A search of the VSLO database—the most widely used third-party database for applying to dermatology away rotations—yielded only 12 (21.4%) of the rotations that we identified in our initial Google search. Similarly, a search of the FARMS database yielded only 17 (30.4%) of the dermatology rotations we previously identified. Aside from missing more than half of the available funded dermatology away rotations, the search process was further complicated by the reliance of the 2 databases on user input rather than presenting all programs offering funded opportunities for dermatology applicants without the need to enter additional information. As of October 26, 2024, there were only 22 inputs for Visiting Scholars Resources across all specialties and programs in the VLSO system.
Our findings indicate a clear need for a reliable and accurate database that captures all funded dermatology rotations for prospective URiM applicants because of the strong emphasis on visiting rotations for application success. Our team created a Google spreadsheet compiling dermatology visiting student health equity and inclusion scholarships from inputs we found in our search. We shared this resource via the Association of Professors of Dermatology listserve so program members could verify the opportunities we compiled to create an accurate and updated resource for finding funded dermatology rotations. The program verification process was conducted by having residency program directors or their respective program coordinators mark “yes” on the spreadsheet to confirm the funded rotation is being offered by their program. Our spreadsheet will continue to be updated yearly through cooperation with participating programs to verify their funded electives and through partnership with the AAMC to include our database in their Visiting Students Resources Database that will be released each year within VLSO as applications open for the following season.
The main limitation of our review is that we presume the information provided in the VSLO and FARMS databases has not changed or been updated to include more programs since our initial search period. Additionally, the information available on dermatology residency program websites limits the data on the total programs obtained, as some website links may not be updated or may be invalid for online web user access. The benefit to creating and continually updating our Dermatology Visiting Student Health Equity and Inclusion Scholarship Database spreadsheet will be to ensure that programs regularly verify their offered funded electives and capture the true total of funded rotations offered for URiM students across the country. We also acknowledge that we did not investigate how URiM student attendance at funded rotations affected their outcomes in matching dermatology programs for residency; however, given the importance of away rotations, which positively influence the ability of URiM students to receive interviews, it is understood that these opportunities are viewed as widely beneficial.
Final Thoughts
The current online search tools that URiM students can use to find funded away rotations in dermatology exclude many of the available opportunities. We aimed to provide an updated and centralized resource for students via the shared spreadsheet we created for residency program directors, but further measures to centralize the most up-to-date information on visiting programs offering scholarships to URiM students would be beneficial.

- Cucka B, Grant-Kels JM. Ethical implications of the high cost of medical student visiting dermatology rotations. Clin Dermatol. 2022;40:539-540. doi:10.1016/j.clindermatol.2022.05.001
- Association of American Medical Colleges. Away rotations of U.S. medical school graduates by intended specialty, 2020 AAMC Medical School Graduation Questionnaire (GQ). Published September 24, 2020. Accessed May 1, 2024. https://students-residents.aamc.org/media/9496/download
- Dahak S, Fernandez JM, Rosman IS. Funded dermatology visiting elective rotations for medical students who are underrepresented in medicine: a cross-sectional analysis. J Am Acad Dermatol. 2023;88: 941-943. doi:10.1016/j.jaad.2022.11.018
- Chen A, Shinkai K. Rethinking how we select dermatology applicants —turning the tide. JAMA Dermatol. 2017;153:259-260. doi:10.1001 /jamadermatol.2016.4683
- Soliman YS, Rzepecki AK, Guzman AK, et al. Understanding perceived barriers of minority medical students pursuing a career in dermatology. JAMA Dermatol. 2019;155:252-254. doi:10.1001 /jamadermatol.2018.4813
- Association of American Medical Colleges. Table B5. Number of active MD residents, by race/ethnicity (alone or in combination) and GME specialty. 2023-24 active residents. Accessed March 8, 2025. https://www.aamc.org/data-reports/students-residents/data/report-residents/2024/table-b5-md-residents-race-ethnicity-and-specialty
- United States Census Bureau. QuickFacts: United States. population estimates, July 1, 2024 (V2024). Accessed February 27, 2025. https://www.census.gov/quickfacts/fact/table/US/PST045221
- El-Kashlan N, Alexis A. Disparities in dermatology: a reflection. J Clin Aesthet Dermatol. 2022;15:27-29.
- Gonzalez S, Syder N, Mckenzie SA, et al. Racial diversity in academic dermatology: a cross-sectional analysis of Black academic dermatology faculty in the United States. J Am Acad Dermatol. 2024;90:182-184. doi:10.1016/j.jaad.2023.09.027
- National Resident Matching Program, Data Release and Research Committee. Results of the 2021 NRMP Program Director Survey, 2021. August 2021. Accessed March 9, 2025. https://www.nrmp.org/wp-content/uploads/2021/11/2021-PD-Survey-Report-for-WWW.pdf
- Winterton M, Ahn J, Bernstein J. The prevalence and cost of medical student visiting rotations. BMC Med Educ. 2016;16:291. doi:10.1186 /s12909-016-0805-z
Most medical students applying to dermatology residency programs in the United States will participate in an away rotation at an outside institution. Prior to COVID-19–related restrictions, 86.7% of dermatology applicants from the class of 2020 reported completing one or more away rotations for their application cycle.1,2 This requirement can be considerably costly, especially since most programs do not offer financial support for travel, living expenses, or housing during these visiting experiences.3 Underrepresented in medicine (URiM) students may be particularly disadvantaged with regard to the financial obligations that come with away rotations.4,5 Visiting scholarships for URiM students can mitigate these challenges, creating opportunities for increasing diversity in dermatology. When medical students begin the residency application process, the Visiting Student Learning Opportunities (VSLO) program of the Association of American Medical Colleges (AAMC) is the most widely used third-party service for submitting applications. For many URiM students, an unforeseen challenge when applying to dermatology residency programs is the lack of an easily accessible and up-to-date search tool to find programs that offer funding, resulting in more time spent searching and thereby complicating the application process. The VSLO released the Visiting Scholars Resources Database, a search tool that aims to compile opportunities for additional support—academic professional, and/or financial—to address this issue. Additionally, the Funded Away Rotations for Minority Medical Students (FARMS) database is an independent directory of programs that offer stipends to URiM students. In this study, we evaluated the efficacy of the VLSO’s Visiting Scholars Resources Database search tool and the FARMS database in identifying funded dermatology rotations for URiM students.
Overview of Online Search Tools
We used the AAMC’s Electronic Residency Application Service Directory to identify 141 programs offering dermatology residency positions. We then conducted a Google search using each program name with the phrase underrepresented in medicine dermatology away rotation to identify any opportunities noted in the Google results offering scholarship funding for URiM students. If there were no Google results for a webpage discussing URiM away rotation opportunities for a certain program, the individual program’s website search box was queried using the terms URiM, scholarship, and funding. If there were no relevant results, the webpages associated with the dermatology department, away rotations, and diversity and inclusion on the respective institution’s website were reviewed to confirm no indication of funded URiM opportunities. Of the 141 dermatology programs we evaluated, we identified 56 (39.7%) that offered funded away rotations for URiM students.
For comparison, we conducted a search of the VSLO’s Visiting Scholars Resources Database to identify programs that listed dermatology, all (specialties), or any (specialties) under the Specialty column that also had a financial resource for URiM students. Our search of the VSLO database yielded only 12 (21.4%) of the 56 funded away rotations we identified via our initial Google and program website search. Program listings tagged for dermatology also were retrieved from the FARMS database, of which only 17 (30.4%) of the 56 funded away rotations we previously identified were included. All queries were performed from October 24 to October 26, 2024 (Figure).

Comment
The 2023-2024 AAMC Report on Residents indicated that 54.9% (800/1455) of active US dermatology medical residents identified as White, 27.5% (400/1455) identified as Asian, 8.9% (129/1455) identified as Hispanic, and 8.7% (126/1455) identified as Black or African American.6 By comparison, 19.5% of the general US population identifies as Hispanic and 13.7% identifies as Black.7 Within the field of dermatology, the proportion of Black dermatology academic faculty in the US is estimated to comprise only 18.7% of all active Black dermatologists.8,9 With a growing population of minority US citizens, the dermatology workforce is lagging in representation across all minority populations, especially when it comes to Hispanic and Black individuals. To increase the diversity of the US dermatology workforce, residency programs must prioritize recruitment of URiM students and support their retention as future faculty.
Reports in the literature suggest that clinical grades, US Medical Licensing Examination scores, letters of recommendation/ networking, and the risk of not matching are among the primary concerns that URiM students face as potential barriers to applying for dermatology residency.4 Meanwhile, dermatology program directors ranked diversity characteristics, perceived interest in the program, personal prior knowledge of an applicant, and audition rotation in their department as important considerations for interviewing applicants.10 As a result, URiM students may have the diverse characteristics that program directors are looking for, but obtaining away rotations and establishing mentors at other institutions may be challenging due to the burden of accruing additional costs for visiting rotations.2,10,11 Other reports have indicated that expanding funded dermatology visiting rotations and promoting national programs such as the American Academy of Dermatology Diversity Mentorship Program (https://www.aad.org/member/career/awards/diversity) or the Skin of Color Society Observership Grant (https://skinofcolorsociety.org/what-we-do/mentorship/observership-grant) can be alternative routes for mentorship and networking.3
Our review demonstrated that, of the 141 dermatology residency programs we identified, only around 40% offer funded rotations for URiM students; however, the current databases that applicants use to find these opportunities do not adequately present the number of available options. A search of the VSLO database—the most widely used third-party database for applying to dermatology away rotations—yielded only 12 (21.4%) of the rotations that we identified in our initial Google search. Similarly, a search of the FARMS database yielded only 17 (30.4%) of the dermatology rotations we previously identified. Aside from missing more than half of the available funded dermatology away rotations, the search process was further complicated by the reliance of the 2 databases on user input rather than presenting all programs offering funded opportunities for dermatology applicants without the need to enter additional information. As of October 26, 2024, there were only 22 inputs for Visiting Scholars Resources across all specialties and programs in the VLSO system.
Our findings indicate a clear need for a reliable and accurate database that captures all funded dermatology rotations for prospective URiM applicants because of the strong emphasis on visiting rotations for application success. Our team created a Google spreadsheet compiling dermatology visiting student health equity and inclusion scholarships from inputs we found in our search. We shared this resource via the Association of Professors of Dermatology listserve so program members could verify the opportunities we compiled to create an accurate and updated resource for finding funded dermatology rotations. The program verification process was conducted by having residency program directors or their respective program coordinators mark “yes” on the spreadsheet to confirm the funded rotation is being offered by their program. Our spreadsheet will continue to be updated yearly through cooperation with participating programs to verify their funded electives and through partnership with the AAMC to include our database in their Visiting Students Resources Database that will be released each year within VLSO as applications open for the following season.
The main limitation of our review is that we presume the information provided in the VSLO and FARMS databases has not changed or been updated to include more programs since our initial search period. Additionally, the information available on dermatology residency program websites limits the data on the total programs obtained, as some website links may not be updated or may be invalid for online web user access. The benefit to creating and continually updating our Dermatology Visiting Student Health Equity and Inclusion Scholarship Database spreadsheet will be to ensure that programs regularly verify their offered funded electives and capture the true total of funded rotations offered for URiM students across the country. We also acknowledge that we did not investigate how URiM student attendance at funded rotations affected their outcomes in matching dermatology programs for residency; however, given the importance of away rotations, which positively influence the ability of URiM students to receive interviews, it is understood that these opportunities are viewed as widely beneficial.
Final Thoughts
The current online search tools that URiM students can use to find funded away rotations in dermatology exclude many of the available opportunities. We aimed to provide an updated and centralized resource for students via the shared spreadsheet we created for residency program directors, but further measures to centralize the most up-to-date information on visiting programs offering scholarships to URiM students would be beneficial.

Most medical students applying to dermatology residency programs in the United States will participate in an away rotation at an outside institution. Prior to COVID-19–related restrictions, 86.7% of dermatology applicants from the class of 2020 reported completing one or more away rotations for their application cycle.1,2 This requirement can be considerably costly, especially since most programs do not offer financial support for travel, living expenses, or housing during these visiting experiences.3 Underrepresented in medicine (URiM) students may be particularly disadvantaged with regard to the financial obligations that come with away rotations.4,5 Visiting scholarships for URiM students can mitigate these challenges, creating opportunities for increasing diversity in dermatology. When medical students begin the residency application process, the Visiting Student Learning Opportunities (VSLO) program of the Association of American Medical Colleges (AAMC) is the most widely used third-party service for submitting applications. For many URiM students, an unforeseen challenge when applying to dermatology residency programs is the lack of an easily accessible and up-to-date search tool to find programs that offer funding, resulting in more time spent searching and thereby complicating the application process. The VSLO released the Visiting Scholars Resources Database, a search tool that aims to compile opportunities for additional support—academic professional, and/or financial—to address this issue. Additionally, the Funded Away Rotations for Minority Medical Students (FARMS) database is an independent directory of programs that offer stipends to URiM students. In this study, we evaluated the efficacy of the VLSO’s Visiting Scholars Resources Database search tool and the FARMS database in identifying funded dermatology rotations for URiM students.
Overview of Online Search Tools
We used the AAMC’s Electronic Residency Application Service Directory to identify 141 programs offering dermatology residency positions. We then conducted a Google search using each program name with the phrase underrepresented in medicine dermatology away rotation to identify any opportunities noted in the Google results offering scholarship funding for URiM students. If there were no Google results for a webpage discussing URiM away rotation opportunities for a certain program, the individual program’s website search box was queried using the terms URiM, scholarship, and funding. If there were no relevant results, the webpages associated with the dermatology department, away rotations, and diversity and inclusion on the respective institution’s website were reviewed to confirm no indication of funded URiM opportunities. Of the 141 dermatology programs we evaluated, we identified 56 (39.7%) that offered funded away rotations for URiM students.
For comparison, we conducted a search of the VSLO’s Visiting Scholars Resources Database to identify programs that listed dermatology, all (specialties), or any (specialties) under the Specialty column that also had a financial resource for URiM students. Our search of the VSLO database yielded only 12 (21.4%) of the 56 funded away rotations we identified via our initial Google and program website search. Program listings tagged for dermatology also were retrieved from the FARMS database, of which only 17 (30.4%) of the 56 funded away rotations we previously identified were included. All queries were performed from October 24 to October 26, 2024 (Figure).

Comment
The 2023-2024 AAMC Report on Residents indicated that 54.9% (800/1455) of active US dermatology medical residents identified as White, 27.5% (400/1455) identified as Asian, 8.9% (129/1455) identified as Hispanic, and 8.7% (126/1455) identified as Black or African American.6 By comparison, 19.5% of the general US population identifies as Hispanic and 13.7% identifies as Black.7 Within the field of dermatology, the proportion of Black dermatology academic faculty in the US is estimated to comprise only 18.7% of all active Black dermatologists.8,9 With a growing population of minority US citizens, the dermatology workforce is lagging in representation across all minority populations, especially when it comes to Hispanic and Black individuals. To increase the diversity of the US dermatology workforce, residency programs must prioritize recruitment of URiM students and support their retention as future faculty.
Reports in the literature suggest that clinical grades, US Medical Licensing Examination scores, letters of recommendation/ networking, and the risk of not matching are among the primary concerns that URiM students face as potential barriers to applying for dermatology residency.4 Meanwhile, dermatology program directors ranked diversity characteristics, perceived interest in the program, personal prior knowledge of an applicant, and audition rotation in their department as important considerations for interviewing applicants.10 As a result, URiM students may have the diverse characteristics that program directors are looking for, but obtaining away rotations and establishing mentors at other institutions may be challenging due to the burden of accruing additional costs for visiting rotations.2,10,11 Other reports have indicated that expanding funded dermatology visiting rotations and promoting national programs such as the American Academy of Dermatology Diversity Mentorship Program (https://www.aad.org/member/career/awards/diversity) or the Skin of Color Society Observership Grant (https://skinofcolorsociety.org/what-we-do/mentorship/observership-grant) can be alternative routes for mentorship and networking.3
Our review demonstrated that, of the 141 dermatology residency programs we identified, only around 40% offer funded rotations for URiM students; however, the current databases that applicants use to find these opportunities do not adequately present the number of available options. A search of the VSLO database—the most widely used third-party database for applying to dermatology away rotations—yielded only 12 (21.4%) of the rotations that we identified in our initial Google search. Similarly, a search of the FARMS database yielded only 17 (30.4%) of the dermatology rotations we previously identified. Aside from missing more than half of the available funded dermatology away rotations, the search process was further complicated by the reliance of the 2 databases on user input rather than presenting all programs offering funded opportunities for dermatology applicants without the need to enter additional information. As of October 26, 2024, there were only 22 inputs for Visiting Scholars Resources across all specialties and programs in the VLSO system.
Our findings indicate a clear need for a reliable and accurate database that captures all funded dermatology rotations for prospective URiM applicants because of the strong emphasis on visiting rotations for application success. Our team created a Google spreadsheet compiling dermatology visiting student health equity and inclusion scholarships from inputs we found in our search. We shared this resource via the Association of Professors of Dermatology listserve so program members could verify the opportunities we compiled to create an accurate and updated resource for finding funded dermatology rotations. The program verification process was conducted by having residency program directors or their respective program coordinators mark “yes” on the spreadsheet to confirm the funded rotation is being offered by their program. Our spreadsheet will continue to be updated yearly through cooperation with participating programs to verify their funded electives and through partnership with the AAMC to include our database in their Visiting Students Resources Database that will be released each year within VLSO as applications open for the following season.
The main limitation of our review is that we presume the information provided in the VSLO and FARMS databases has not changed or been updated to include more programs since our initial search period. Additionally, the information available on dermatology residency program websites limits the data on the total programs obtained, as some website links may not be updated or may be invalid for online web user access. The benefit to creating and continually updating our Dermatology Visiting Student Health Equity and Inclusion Scholarship Database spreadsheet will be to ensure that programs regularly verify their offered funded electives and capture the true total of funded rotations offered for URiM students across the country. We also acknowledge that we did not investigate how URiM student attendance at funded rotations affected their outcomes in matching dermatology programs for residency; however, given the importance of away rotations, which positively influence the ability of URiM students to receive interviews, it is understood that these opportunities are viewed as widely beneficial.
Final Thoughts
The current online search tools that URiM students can use to find funded away rotations in dermatology exclude many of the available opportunities. We aimed to provide an updated and centralized resource for students via the shared spreadsheet we created for residency program directors, but further measures to centralize the most up-to-date information on visiting programs offering scholarships to URiM students would be beneficial.

- Cucka B, Grant-Kels JM. Ethical implications of the high cost of medical student visiting dermatology rotations. Clin Dermatol. 2022;40:539-540. doi:10.1016/j.clindermatol.2022.05.001
- Association of American Medical Colleges. Away rotations of U.S. medical school graduates by intended specialty, 2020 AAMC Medical School Graduation Questionnaire (GQ). Published September 24, 2020. Accessed May 1, 2024. https://students-residents.aamc.org/media/9496/download
- Dahak S, Fernandez JM, Rosman IS. Funded dermatology visiting elective rotations for medical students who are underrepresented in medicine: a cross-sectional analysis. J Am Acad Dermatol. 2023;88: 941-943. doi:10.1016/j.jaad.2022.11.018
- Chen A, Shinkai K. Rethinking how we select dermatology applicants —turning the tide. JAMA Dermatol. 2017;153:259-260. doi:10.1001 /jamadermatol.2016.4683
- Soliman YS, Rzepecki AK, Guzman AK, et al. Understanding perceived barriers of minority medical students pursuing a career in dermatology. JAMA Dermatol. 2019;155:252-254. doi:10.1001 /jamadermatol.2018.4813
- Association of American Medical Colleges. Table B5. Number of active MD residents, by race/ethnicity (alone or in combination) and GME specialty. 2023-24 active residents. Accessed March 8, 2025. https://www.aamc.org/data-reports/students-residents/data/report-residents/2024/table-b5-md-residents-race-ethnicity-and-specialty
- United States Census Bureau. QuickFacts: United States. population estimates, July 1, 2024 (V2024). Accessed February 27, 2025. https://www.census.gov/quickfacts/fact/table/US/PST045221
- El-Kashlan N, Alexis A. Disparities in dermatology: a reflection. J Clin Aesthet Dermatol. 2022;15:27-29.
- Gonzalez S, Syder N, Mckenzie SA, et al. Racial diversity in academic dermatology: a cross-sectional analysis of Black academic dermatology faculty in the United States. J Am Acad Dermatol. 2024;90:182-184. doi:10.1016/j.jaad.2023.09.027
- National Resident Matching Program, Data Release and Research Committee. Results of the 2021 NRMP Program Director Survey, 2021. August 2021. Accessed March 9, 2025. https://www.nrmp.org/wp-content/uploads/2021/11/2021-PD-Survey-Report-for-WWW.pdf
- Winterton M, Ahn J, Bernstein J. The prevalence and cost of medical student visiting rotations. BMC Med Educ. 2016;16:291. doi:10.1186 /s12909-016-0805-z
- Cucka B, Grant-Kels JM. Ethical implications of the high cost of medical student visiting dermatology rotations. Clin Dermatol. 2022;40:539-540. doi:10.1016/j.clindermatol.2022.05.001
- Association of American Medical Colleges. Away rotations of U.S. medical school graduates by intended specialty, 2020 AAMC Medical School Graduation Questionnaire (GQ). Published September 24, 2020. Accessed May 1, 2024. https://students-residents.aamc.org/media/9496/download
- Dahak S, Fernandez JM, Rosman IS. Funded dermatology visiting elective rotations for medical students who are underrepresented in medicine: a cross-sectional analysis. J Am Acad Dermatol. 2023;88: 941-943. doi:10.1016/j.jaad.2022.11.018
- Chen A, Shinkai K. Rethinking how we select dermatology applicants —turning the tide. JAMA Dermatol. 2017;153:259-260. doi:10.1001 /jamadermatol.2016.4683
- Soliman YS, Rzepecki AK, Guzman AK, et al. Understanding perceived barriers of minority medical students pursuing a career in dermatology. JAMA Dermatol. 2019;155:252-254. doi:10.1001 /jamadermatol.2018.4813
- Association of American Medical Colleges. Table B5. Number of active MD residents, by race/ethnicity (alone or in combination) and GME specialty. 2023-24 active residents. Accessed March 8, 2025. https://www.aamc.org/data-reports/students-residents/data/report-residents/2024/table-b5-md-residents-race-ethnicity-and-specialty
- United States Census Bureau. QuickFacts: United States. population estimates, July 1, 2024 (V2024). Accessed February 27, 2025. https://www.census.gov/quickfacts/fact/table/US/PST045221
- El-Kashlan N, Alexis A. Disparities in dermatology: a reflection. J Clin Aesthet Dermatol. 2022;15:27-29.
- Gonzalez S, Syder N, Mckenzie SA, et al. Racial diversity in academic dermatology: a cross-sectional analysis of Black academic dermatology faculty in the United States. J Am Acad Dermatol. 2024;90:182-184. doi:10.1016/j.jaad.2023.09.027
- National Resident Matching Program, Data Release and Research Committee. Results of the 2021 NRMP Program Director Survey, 2021. August 2021. Accessed March 9, 2025. https://www.nrmp.org/wp-content/uploads/2021/11/2021-PD-Survey-Report-for-WWW.pdf
- Winterton M, Ahn J, Bernstein J. The prevalence and cost of medical student visiting rotations. BMC Med Educ. 2016;16:291. doi:10.1186 /s12909-016-0805-z
A Review of Online Search Tools to Identify Funded Dermatology Away Rotations for Underrepresented Medical Students
A Review of Online Search Tools to Identify Funded Dermatology Away Rotations for Underrepresented Medical Students
PRACTICE POINTS
- Many funded away rotations are not listed on the most widely used databases for applying to dermatology residency programs.
- Underrepresented in medicine students who are seeking funded dermatology away rotations would benefit from a centralized, comprehensive, and well-organized database to improve equity of opportunity in the dermatology rotation application search process and further diversify the specialty.
- There are limited data assessing outcomes associated with participation in funded rotation and residency match outcomes.
Key Features of Dermatosis Papulosa Nigra vs Seborrheic Keratosis
Key Features of Dermatosis Papulosa Nigra vs Seborrheic Keratosis
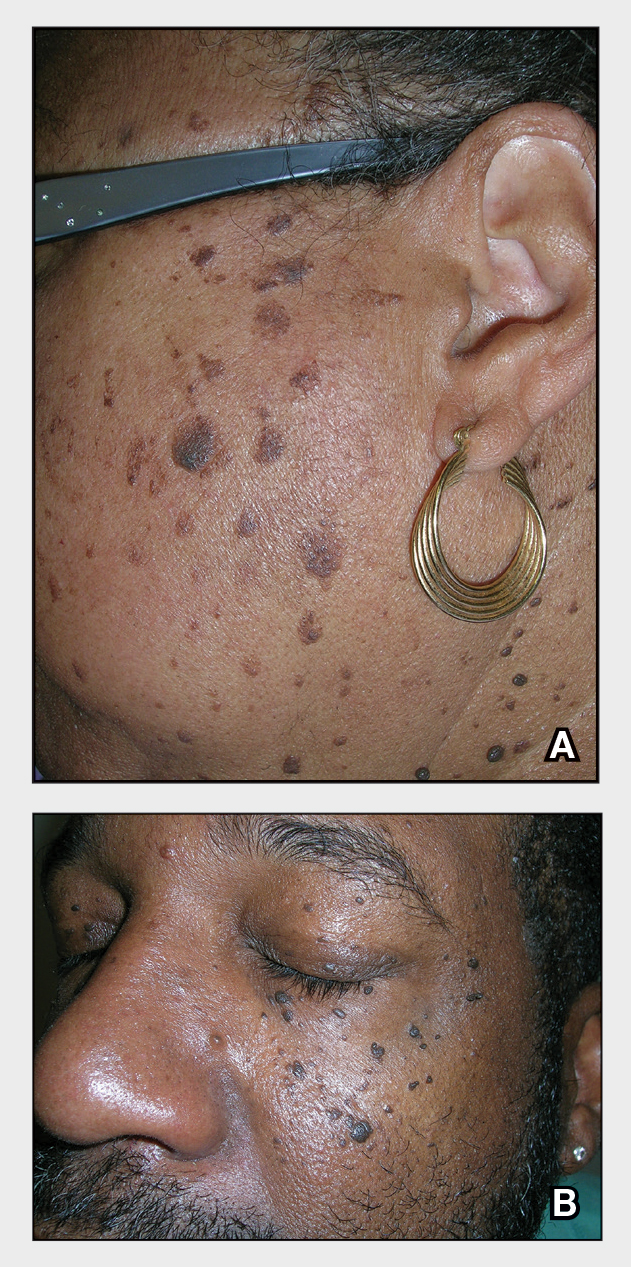
THE COMPARISON
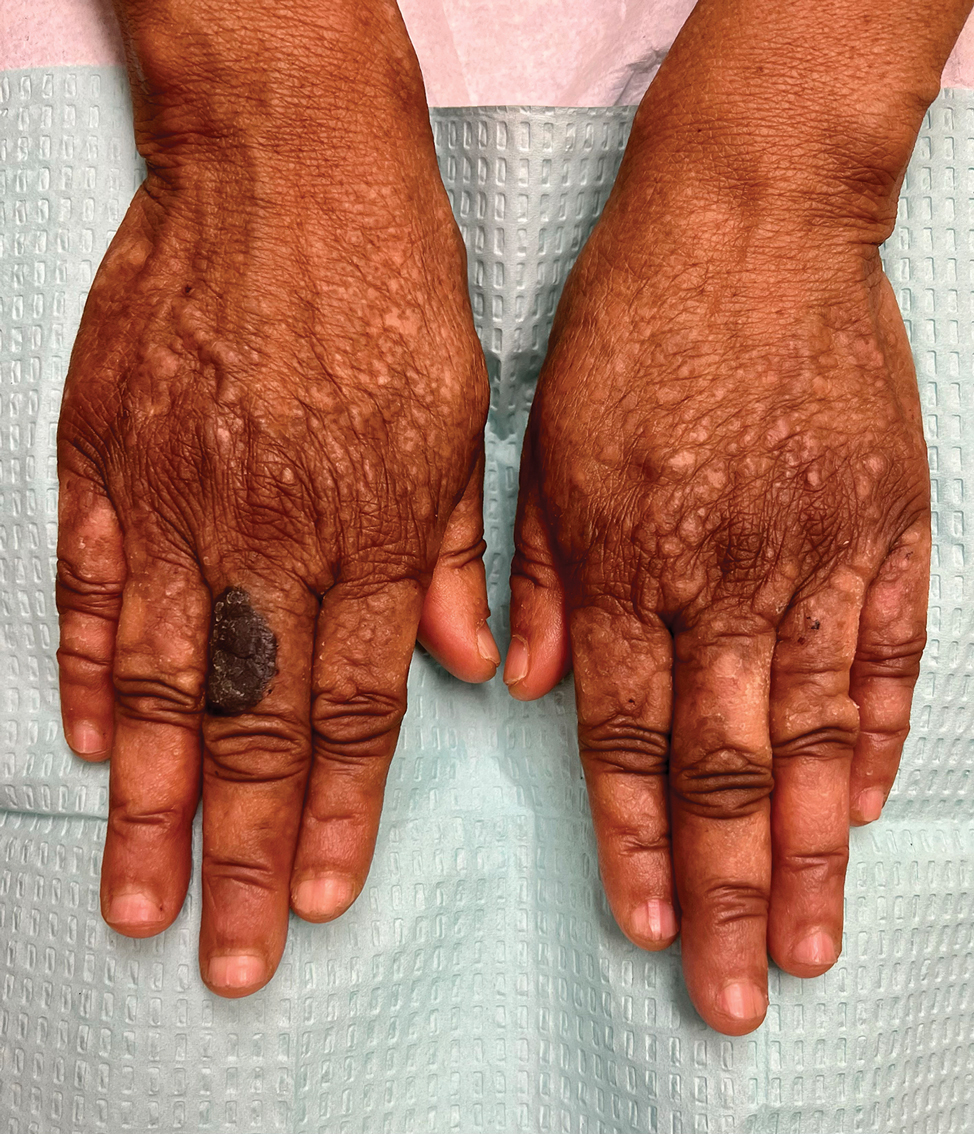
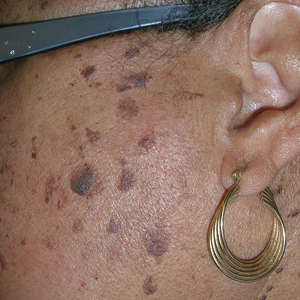
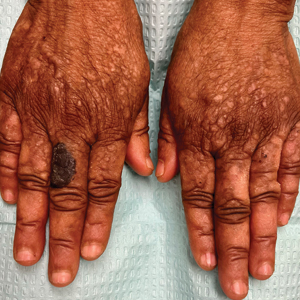
- A A Black woman with dermatosis papulosa nigra manifesting as a cluster of light brown flat seborrheic keratoses that covered the cheeks and lateral face and extended to the neck.
- B A Black man with dermatosis papulosa nigra manifesting as small black papules on the cheeks and eyelids involving the central face.
Dermatosis papulosa nigra (DPN), a subvariant of seborrheic keratosis (SK), is characterized by benign pigmented epidermal neoplasms that typically manifest on the face, neck, and trunk in individuals with darker skin tones.1,2 While DPN meets the diagnostic criteria for SK, certain characteristics can help distinguish these lesions from other SK types. Treatment of DPN in patients with skin of color requires caution, particularly regarding the use of abrasive methods as well as cryotherapy, which generally should be avoided.
Epidemiology
The incidence of SKs increases with age.3,4 Although it can occur in patients of all skin tones, SK is more common in lighter skin tones, while DPN predominantly is diagnosed in darker skin types.1,4 The prevalence of DPN in Black patients ranges from 10% to 30%, and Black women are twice as likely to be diagnosed with DPN as men.2 One study reported a first-degree relative with DPN in 84% (42/50) of patients.5 The number and size of DPN papules increase with age.1
Key Clinical Features
Dermatosis papulosa nigra and SK have distinctive morphologies: DPN typically manifests as raised, round or filiform, sessile, brown to black, 1- to 5-mm papules.2 Seborrheic keratoses tend to be larger with a “stuck on” appearance and manifest as well-demarcated, pink to black papules or plaques that can range in size from millimeters to a few centimeters.3,4 In DPN, the lesions usually are asymptomatic but may be tender, pruritic, dry, or scaly and may become irritated.1,2 They develop symmetrically in sun-exposed areas, and the most common sites are the malar face, temporal region, neck, and trunk.1,2,6,7 Seborrheic keratoses can appear throughout the body, including in sun-exposed areas, but have varying textures (eg, greasy, waxy, verrucous).3,4
Worth Noting
Dermatosis papulosa nigra and SK can resemble each other histologically: DPN demonstrates a fibrous stroma, papillomatosis, hyperkeratosis, and acanthosis at the intraepidermal layer, which are diagnostic criteria for SK.2,4,8 However, other histologic features characteristic of SK that are not seen in DPN include pseudohorn cysts, spindle tumor cells, and basaloid cell nests.8
Dermoscopy can be useful in ruling out malignant skin cancers when evaluating pigmented lesions. The most common dermoscopic features of SK are cerebriform patterns such as fissures and ridges, comedolike openings, and pigmented fingerprintlike structures.3,4 To a lesser degree, milialike cysts, sharp demarcation, and hairpin-shaped vascular structures also may be present.4 The dermoscopic findings of DPN have not been well evaluated, but one study revealed that DPN had similar dermoscopic features to SK with some predominant features.6 Ridges and fissures were seen in 59% of patients diagnosed with DPN followed by comedolike openings seen in 27% of patients. The coexistence of a cerebriform pattern with comedolike openings was infrequent, and milialike cysts were rare.6
While DPN and SK are benign, patients often seek treatment for cosmetic reasons. Factors to consider when choosing a treatment modality include location of the lesions, the patient’s skin tone, and postprocedural outcomes (eg, depigmentation, wound healing). In general, treatments for SK include cryotherapy, electrodesiccation and curettage, and topical therapeutics such as hydrogen peroxide 40%, topical vitamin D3, and nitric-zinc 30%-50% solutions.4,8 Well-established treatment options for DPN include electrodesiccation, laser therapies, scissor excision, and cryotherapy, but topical options such as tazarotene also have been reported.1,9 Of the treatments for DPN, electrodesiccation and laser therapy routinely are used.10
The efficacy of electrodessication and potassium titanyl phosphate (KTP) laser were assessed in a randomized, investigator-blinded split-face study.11 Both modalities received high improvement ratings, with the results favoring the KTP laser. The patients (most of whom were Black) reported that KTP laser was more effective but more painful than electrodessication (P=.002).11 In another randomized study, patients received 3 treatments—electrodessication, pulsed dye laser, and curettage—for select DPN papules.10 There was no difference in the degree of clearance, cosmetic outcome, or postinflammatory hyperpigmentation between the 3 modalities, but patients found the laser to be the most painful.
It is important to exercise caution when using abrasive methods (eg, laser therapy, electrodesiccation, curettage) in patients with darker skin tones because of the increased risk for postinflammatory pigment alteration.1,2,12 Adverse effects of treatment are a top concern in the management of DPN.5,13 While cryotherapy is a preferred treatment of SK in lighter skin tones, it generally is avoided for DPN in darker skin types because melanocyte destruction can lead to cosmetically unsatisfactory and easily visible depigmentation.9
To mitigate postprocedural adverse effects, proper aftercare can promote wound healing and minimize postinflammatory pigment alteration. In one split-face study of Black patients, 2 DPN papules were removed from each side of the face using fine-curved surgical scissors.14 Next, a petrolatum-based ointment and an antibiotic ointment with polymyxin B sulfate/bacitracin zinc was applied twice daily for 21 days to opposite sides of the face. Patients did not develop infection, tolerated both treatments well, and demonstrated improved general wound appearance according to investigator- rated clinical assessment.14 Other reported postprocedural approaches include using topical agents with ingredients shown to improve hyperpigmentation (eg, niacinamide, azelaic acid) as well as photoprotection.12
Health Disparity Highlight
While DPN is benign, it can have adverse psychosocial effects on patients. A study in Senegal revealed that 60% (19/30) of patients with DPN experienced anxiety related to their condition, while others noted that DPN hindered their social relationships.13 In one US study of 50 Black patients with DPN, there was a moderate effect on quality of life, and 36% (18/50) of patients had the lesions removed. However, of the treated patients, 67% (12/18) reported few—if any—symptoms prior to removal.5 Although treatment of DPN is widely considered a cosmetic procedure, therapeutic management can address—and may improve—mental health in patients with skin of color.1,5,13 Despite the high prevalence of DPN in patients with darker skin tones, data on treatment frequency and insurance coverage are not widely available, thus limiting our understanding of treatment accessibility and economic burden.
- Frazier WT, Proddutur S, Swope K. Common dermatologic conditions in skin of color. Am Fam Physician.2023;107:26-34.
- Metin SA, Lee BW, Lambert WC, et al. Dermatosis papulosa nigra: a clinically and histopathologically distinct entity. Clin Dermatol. 2017;35:491-496.
- Braun RP, Ludwig S, Marghoob AA. Differential diagnosis of seborrheic keratosis: clinical and dermoscopic features. J Drugs Dermatol. 2017; 16: 835-842.
- Sun MD, Halpern AC. Advances in the etiology, detection, and clinical management of seborrheic keratoses. Dermatology. 2022;238:205-217.
- Uwakwe LN, De Souza B, Subash J, et al. Dermatosis papulosa nigra: a quality of life survey study. J Clin Aesthet Dermatol. 2020;13:17-19.
- Bhat RM, Patrao N, Monteiro R, et al. A clinical, dermoscopic, and histopathological study of dermatosis papulosa nigra (DPN)—an Indian perspective. Int J Dermatol. 2017;56:957-960.
- Karampinis E, Georgopoulou KE, Kampra E, et al. Clinical and dermoscopic patterns of basal cell carcinoma and its mimickers in skin of color: a practical summary. Medicina (Kaunas). 2024;60:1386.
- Gorai S, Ahmad S, Raza SSM, et al. Update of pathophysiology and treatment options of seborrheic keratosis. Dermatol Ther. 2022;35:E15934.
- Jain S, Caire H, Haas CJ. Management of dermatosis papulosa nigra: a systematic review. Int J Dermatol. Published online October 4, 2024.
- Garcia MS, Azari R, Eisen DB. Treatment of dermatosis papulosa nigra in 10 patients: a comparison trial of electrodesiccation, pulsed dye laser, and curettage. Dermatol Surg. 2010;36:1968-1972.
- Kundu RV, Joshi SS, Suh KY, et al. Comparison of electrodesiccation and potassium-titanyl-phosphate laser for treatment of dermatosis papulosa nigra. Dermatol Surg. 2009;35:1079-1083.
- Markiewicz E, Karaman-Jurukovska N, Mammone T, et al. Postinflammatory hyperpigmentation in dark skin: molecular mechanism and skincare implications. Clin Cosmet Investig Dermatol. 2022;15: 2555-2565.
- Niang SO, Kane A, Diallo M, et al. Dermatosis papulosa nigra in Dakar, Senegal. Int J Dermatol. 2007;46(suppl 1):45-47.
- Taylor SC, Averyhart AN, Heath CR. Postprocedural wound-healing efficacy following removal of dermatosis papulosa nigra lesions in an African American population: a comparison of a skin protectant ointment and a topical antibiotic. J Am Acad Dermatol. 2011;64(suppl 3):S30-S35.

THE COMPARISON
- A A Black woman with dermatosis papulosa nigra manifesting as a cluster of light brown flat seborrheic keratoses that covered the cheeks and lateral face and extended to the neck.
- B A Black man with dermatosis papulosa nigra manifesting as small black papules on the cheeks and eyelids involving the central face.
Dermatosis papulosa nigra (DPN), a subvariant of seborrheic keratosis (SK), is characterized by benign pigmented epidermal neoplasms that typically manifest on the face, neck, and trunk in individuals with darker skin tones.1,2 While DPN meets the diagnostic criteria for SK, certain characteristics can help distinguish these lesions from other SK types. Treatment of DPN in patients with skin of color requires caution, particularly regarding the use of abrasive methods as well as cryotherapy, which generally should be avoided.
Epidemiology
The incidence of SKs increases with age.3,4 Although it can occur in patients of all skin tones, SK is more common in lighter skin tones, while DPN predominantly is diagnosed in darker skin types.1,4 The prevalence of DPN in Black patients ranges from 10% to 30%, and Black women are twice as likely to be diagnosed with DPN as men.2 One study reported a first-degree relative with DPN in 84% (42/50) of patients.5 The number and size of DPN papules increase with age.1
Key Clinical Features
Dermatosis papulosa nigra and SK have distinctive morphologies: DPN typically manifests as raised, round or filiform, sessile, brown to black, 1- to 5-mm papules.2 Seborrheic keratoses tend to be larger with a “stuck on” appearance and manifest as well-demarcated, pink to black papules or plaques that can range in size from millimeters to a few centimeters.3,4 In DPN, the lesions usually are asymptomatic but may be tender, pruritic, dry, or scaly and may become irritated.1,2 They develop symmetrically in sun-exposed areas, and the most common sites are the malar face, temporal region, neck, and trunk.1,2,6,7 Seborrheic keratoses can appear throughout the body, including in sun-exposed areas, but have varying textures (eg, greasy, waxy, verrucous).3,4
Worth Noting
Dermatosis papulosa nigra and SK can resemble each other histologically: DPN demonstrates a fibrous stroma, papillomatosis, hyperkeratosis, and acanthosis at the intraepidermal layer, which are diagnostic criteria for SK.2,4,8 However, other histologic features characteristic of SK that are not seen in DPN include pseudohorn cysts, spindle tumor cells, and basaloid cell nests.8
Dermoscopy can be useful in ruling out malignant skin cancers when evaluating pigmented lesions. The most common dermoscopic features of SK are cerebriform patterns such as fissures and ridges, comedolike openings, and pigmented fingerprintlike structures.3,4 To a lesser degree, milialike cysts, sharp demarcation, and hairpin-shaped vascular structures also may be present.4 The dermoscopic findings of DPN have not been well evaluated, but one study revealed that DPN had similar dermoscopic features to SK with some predominant features.6 Ridges and fissures were seen in 59% of patients diagnosed with DPN followed by comedolike openings seen in 27% of patients. The coexistence of a cerebriform pattern with comedolike openings was infrequent, and milialike cysts were rare.6
While DPN and SK are benign, patients often seek treatment for cosmetic reasons. Factors to consider when choosing a treatment modality include location of the lesions, the patient’s skin tone, and postprocedural outcomes (eg, depigmentation, wound healing). In general, treatments for SK include cryotherapy, electrodesiccation and curettage, and topical therapeutics such as hydrogen peroxide 40%, topical vitamin D3, and nitric-zinc 30%-50% solutions.4,8 Well-established treatment options for DPN include electrodesiccation, laser therapies, scissor excision, and cryotherapy, but topical options such as tazarotene also have been reported.1,9 Of the treatments for DPN, electrodesiccation and laser therapy routinely are used.10
The efficacy of electrodessication and potassium titanyl phosphate (KTP) laser were assessed in a randomized, investigator-blinded split-face study.11 Both modalities received high improvement ratings, with the results favoring the KTP laser. The patients (most of whom were Black) reported that KTP laser was more effective but more painful than electrodessication (P=.002).11 In another randomized study, patients received 3 treatments—electrodessication, pulsed dye laser, and curettage—for select DPN papules.10 There was no difference in the degree of clearance, cosmetic outcome, or postinflammatory hyperpigmentation between the 3 modalities, but patients found the laser to be the most painful.
It is important to exercise caution when using abrasive methods (eg, laser therapy, electrodesiccation, curettage) in patients with darker skin tones because of the increased risk for postinflammatory pigment alteration.1,2,12 Adverse effects of treatment are a top concern in the management of DPN.5,13 While cryotherapy is a preferred treatment of SK in lighter skin tones, it generally is avoided for DPN in darker skin types because melanocyte destruction can lead to cosmetically unsatisfactory and easily visible depigmentation.9
To mitigate postprocedural adverse effects, proper aftercare can promote wound healing and minimize postinflammatory pigment alteration. In one split-face study of Black patients, 2 DPN papules were removed from each side of the face using fine-curved surgical scissors.14 Next, a petrolatum-based ointment and an antibiotic ointment with polymyxin B sulfate/bacitracin zinc was applied twice daily for 21 days to opposite sides of the face. Patients did not develop infection, tolerated both treatments well, and demonstrated improved general wound appearance according to investigator- rated clinical assessment.14 Other reported postprocedural approaches include using topical agents with ingredients shown to improve hyperpigmentation (eg, niacinamide, azelaic acid) as well as photoprotection.12
Health Disparity Highlight
While DPN is benign, it can have adverse psychosocial effects on patients. A study in Senegal revealed that 60% (19/30) of patients with DPN experienced anxiety related to their condition, while others noted that DPN hindered their social relationships.13 In one US study of 50 Black patients with DPN, there was a moderate effect on quality of life, and 36% (18/50) of patients had the lesions removed. However, of the treated patients, 67% (12/18) reported few—if any—symptoms prior to removal.5 Although treatment of DPN is widely considered a cosmetic procedure, therapeutic management can address—and may improve—mental health in patients with skin of color.1,5,13 Despite the high prevalence of DPN in patients with darker skin tones, data on treatment frequency and insurance coverage are not widely available, thus limiting our understanding of treatment accessibility and economic burden.

THE COMPARISON
- A A Black woman with dermatosis papulosa nigra manifesting as a cluster of light brown flat seborrheic keratoses that covered the cheeks and lateral face and extended to the neck.
- B A Black man with dermatosis papulosa nigra manifesting as small black papules on the cheeks and eyelids involving the central face.
Dermatosis papulosa nigra (DPN), a subvariant of seborrheic keratosis (SK), is characterized by benign pigmented epidermal neoplasms that typically manifest on the face, neck, and trunk in individuals with darker skin tones.1,2 While DPN meets the diagnostic criteria for SK, certain characteristics can help distinguish these lesions from other SK types. Treatment of DPN in patients with skin of color requires caution, particularly regarding the use of abrasive methods as well as cryotherapy, which generally should be avoided.
Epidemiology
The incidence of SKs increases with age.3,4 Although it can occur in patients of all skin tones, SK is more common in lighter skin tones, while DPN predominantly is diagnosed in darker skin types.1,4 The prevalence of DPN in Black patients ranges from 10% to 30%, and Black women are twice as likely to be diagnosed with DPN as men.2 One study reported a first-degree relative with DPN in 84% (42/50) of patients.5 The number and size of DPN papules increase with age.1
Key Clinical Features
Dermatosis papulosa nigra and SK have distinctive morphologies: DPN typically manifests as raised, round or filiform, sessile, brown to black, 1- to 5-mm papules.2 Seborrheic keratoses tend to be larger with a “stuck on” appearance and manifest as well-demarcated, pink to black papules or plaques that can range in size from millimeters to a few centimeters.3,4 In DPN, the lesions usually are asymptomatic but may be tender, pruritic, dry, or scaly and may become irritated.1,2 They develop symmetrically in sun-exposed areas, and the most common sites are the malar face, temporal region, neck, and trunk.1,2,6,7 Seborrheic keratoses can appear throughout the body, including in sun-exposed areas, but have varying textures (eg, greasy, waxy, verrucous).3,4
Worth Noting
Dermatosis papulosa nigra and SK can resemble each other histologically: DPN demonstrates a fibrous stroma, papillomatosis, hyperkeratosis, and acanthosis at the intraepidermal layer, which are diagnostic criteria for SK.2,4,8 However, other histologic features characteristic of SK that are not seen in DPN include pseudohorn cysts, spindle tumor cells, and basaloid cell nests.8
Dermoscopy can be useful in ruling out malignant skin cancers when evaluating pigmented lesions. The most common dermoscopic features of SK are cerebriform patterns such as fissures and ridges, comedolike openings, and pigmented fingerprintlike structures.3,4 To a lesser degree, milialike cysts, sharp demarcation, and hairpin-shaped vascular structures also may be present.4 The dermoscopic findings of DPN have not been well evaluated, but one study revealed that DPN had similar dermoscopic features to SK with some predominant features.6 Ridges and fissures were seen in 59% of patients diagnosed with DPN followed by comedolike openings seen in 27% of patients. The coexistence of a cerebriform pattern with comedolike openings was infrequent, and milialike cysts were rare.6
While DPN and SK are benign, patients often seek treatment for cosmetic reasons. Factors to consider when choosing a treatment modality include location of the lesions, the patient’s skin tone, and postprocedural outcomes (eg, depigmentation, wound healing). In general, treatments for SK include cryotherapy, electrodesiccation and curettage, and topical therapeutics such as hydrogen peroxide 40%, topical vitamin D3, and nitric-zinc 30%-50% solutions.4,8 Well-established treatment options for DPN include electrodesiccation, laser therapies, scissor excision, and cryotherapy, but topical options such as tazarotene also have been reported.1,9 Of the treatments for DPN, electrodesiccation and laser therapy routinely are used.10
The efficacy of electrodessication and potassium titanyl phosphate (KTP) laser were assessed in a randomized, investigator-blinded split-face study.11 Both modalities received high improvement ratings, with the results favoring the KTP laser. The patients (most of whom were Black) reported that KTP laser was more effective but more painful than electrodessication (P=.002).11 In another randomized study, patients received 3 treatments—electrodessication, pulsed dye laser, and curettage—for select DPN papules.10 There was no difference in the degree of clearance, cosmetic outcome, or postinflammatory hyperpigmentation between the 3 modalities, but patients found the laser to be the most painful.
It is important to exercise caution when using abrasive methods (eg, laser therapy, electrodesiccation, curettage) in patients with darker skin tones because of the increased risk for postinflammatory pigment alteration.1,2,12 Adverse effects of treatment are a top concern in the management of DPN.5,13 While cryotherapy is a preferred treatment of SK in lighter skin tones, it generally is avoided for DPN in darker skin types because melanocyte destruction can lead to cosmetically unsatisfactory and easily visible depigmentation.9
To mitigate postprocedural adverse effects, proper aftercare can promote wound healing and minimize postinflammatory pigment alteration. In one split-face study of Black patients, 2 DPN papules were removed from each side of the face using fine-curved surgical scissors.14 Next, a petrolatum-based ointment and an antibiotic ointment with polymyxin B sulfate/bacitracin zinc was applied twice daily for 21 days to opposite sides of the face. Patients did not develop infection, tolerated both treatments well, and demonstrated improved general wound appearance according to investigator- rated clinical assessment.14 Other reported postprocedural approaches include using topical agents with ingredients shown to improve hyperpigmentation (eg, niacinamide, azelaic acid) as well as photoprotection.12
Health Disparity Highlight
While DPN is benign, it can have adverse psychosocial effects on patients. A study in Senegal revealed that 60% (19/30) of patients with DPN experienced anxiety related to their condition, while others noted that DPN hindered their social relationships.13 In one US study of 50 Black patients with DPN, there was a moderate effect on quality of life, and 36% (18/50) of patients had the lesions removed. However, of the treated patients, 67% (12/18) reported few—if any—symptoms prior to removal.5 Although treatment of DPN is widely considered a cosmetic procedure, therapeutic management can address—and may improve—mental health in patients with skin of color.1,5,13 Despite the high prevalence of DPN in patients with darker skin tones, data on treatment frequency and insurance coverage are not widely available, thus limiting our understanding of treatment accessibility and economic burden.
- Frazier WT, Proddutur S, Swope K. Common dermatologic conditions in skin of color. Am Fam Physician.2023;107:26-34.
- Metin SA, Lee BW, Lambert WC, et al. Dermatosis papulosa nigra: a clinically and histopathologically distinct entity. Clin Dermatol. 2017;35:491-496.
- Braun RP, Ludwig S, Marghoob AA. Differential diagnosis of seborrheic keratosis: clinical and dermoscopic features. J Drugs Dermatol. 2017; 16: 835-842.
- Sun MD, Halpern AC. Advances in the etiology, detection, and clinical management of seborrheic keratoses. Dermatology. 2022;238:205-217.
- Uwakwe LN, De Souza B, Subash J, et al. Dermatosis papulosa nigra: a quality of life survey study. J Clin Aesthet Dermatol. 2020;13:17-19.
- Bhat RM, Patrao N, Monteiro R, et al. A clinical, dermoscopic, and histopathological study of dermatosis papulosa nigra (DPN)—an Indian perspective. Int J Dermatol. 2017;56:957-960.
- Karampinis E, Georgopoulou KE, Kampra E, et al. Clinical and dermoscopic patterns of basal cell carcinoma and its mimickers in skin of color: a practical summary. Medicina (Kaunas). 2024;60:1386.
- Gorai S, Ahmad S, Raza SSM, et al. Update of pathophysiology and treatment options of seborrheic keratosis. Dermatol Ther. 2022;35:E15934.
- Jain S, Caire H, Haas CJ. Management of dermatosis papulosa nigra: a systematic review. Int J Dermatol. Published online October 4, 2024.
- Garcia MS, Azari R, Eisen DB. Treatment of dermatosis papulosa nigra in 10 patients: a comparison trial of electrodesiccation, pulsed dye laser, and curettage. Dermatol Surg. 2010;36:1968-1972.
- Kundu RV, Joshi SS, Suh KY, et al. Comparison of electrodesiccation and potassium-titanyl-phosphate laser for treatment of dermatosis papulosa nigra. Dermatol Surg. 2009;35:1079-1083.
- Markiewicz E, Karaman-Jurukovska N, Mammone T, et al. Postinflammatory hyperpigmentation in dark skin: molecular mechanism and skincare implications. Clin Cosmet Investig Dermatol. 2022;15: 2555-2565.
- Niang SO, Kane A, Diallo M, et al. Dermatosis papulosa nigra in Dakar, Senegal. Int J Dermatol. 2007;46(suppl 1):45-47.
- Taylor SC, Averyhart AN, Heath CR. Postprocedural wound-healing efficacy following removal of dermatosis papulosa nigra lesions in an African American population: a comparison of a skin protectant ointment and a topical antibiotic. J Am Acad Dermatol. 2011;64(suppl 3):S30-S35.
- Frazier WT, Proddutur S, Swope K. Common dermatologic conditions in skin of color. Am Fam Physician.2023;107:26-34.
- Metin SA, Lee BW, Lambert WC, et al. Dermatosis papulosa nigra: a clinically and histopathologically distinct entity. Clin Dermatol. 2017;35:491-496.
- Braun RP, Ludwig S, Marghoob AA. Differential diagnosis of seborrheic keratosis: clinical and dermoscopic features. J Drugs Dermatol. 2017; 16: 835-842.
- Sun MD, Halpern AC. Advances in the etiology, detection, and clinical management of seborrheic keratoses. Dermatology. 2022;238:205-217.
- Uwakwe LN, De Souza B, Subash J, et al. Dermatosis papulosa nigra: a quality of life survey study. J Clin Aesthet Dermatol. 2020;13:17-19.
- Bhat RM, Patrao N, Monteiro R, et al. A clinical, dermoscopic, and histopathological study of dermatosis papulosa nigra (DPN)—an Indian perspective. Int J Dermatol. 2017;56:957-960.
- Karampinis E, Georgopoulou KE, Kampra E, et al. Clinical and dermoscopic patterns of basal cell carcinoma and its mimickers in skin of color: a practical summary. Medicina (Kaunas). 2024;60:1386.
- Gorai S, Ahmad S, Raza SSM, et al. Update of pathophysiology and treatment options of seborrheic keratosis. Dermatol Ther. 2022;35:E15934.
- Jain S, Caire H, Haas CJ. Management of dermatosis papulosa nigra: a systematic review. Int J Dermatol. Published online October 4, 2024.
- Garcia MS, Azari R, Eisen DB. Treatment of dermatosis papulosa nigra in 10 patients: a comparison trial of electrodesiccation, pulsed dye laser, and curettage. Dermatol Surg. 2010;36:1968-1972.
- Kundu RV, Joshi SS, Suh KY, et al. Comparison of electrodesiccation and potassium-titanyl-phosphate laser for treatment of dermatosis papulosa nigra. Dermatol Surg. 2009;35:1079-1083.
- Markiewicz E, Karaman-Jurukovska N, Mammone T, et al. Postinflammatory hyperpigmentation in dark skin: molecular mechanism and skincare implications. Clin Cosmet Investig Dermatol. 2022;15: 2555-2565.
- Niang SO, Kane A, Diallo M, et al. Dermatosis papulosa nigra in Dakar, Senegal. Int J Dermatol. 2007;46(suppl 1):45-47.
- Taylor SC, Averyhart AN, Heath CR. Postprocedural wound-healing efficacy following removal of dermatosis papulosa nigra lesions in an African American population: a comparison of a skin protectant ointment and a topical antibiotic. J Am Acad Dermatol. 2011;64(suppl 3):S30-S35.
Key Features of Dermatosis Papulosa Nigra vs Seborrheic Keratosis
Key Features of Dermatosis Papulosa Nigra vs Seborrheic Keratosis
Treatment of Seborrheic Dermatitis in Black Patients
Treatment of Seborrheic Dermatitis in Black Patients
Seborrheic dermatitis (SD) is a common chronic inflammatory skin condition that predominantly affects areas with high concentrations of sebaceous glands such as the scalp and face. Up to 5% of the worldwide population is affected by SD each year, causing a major burden of disease for patients and the health care system.1 In 2023, the cost of medical treatment for SD in the United States was $300 million, with outpatient office visits alone costing $58 million and prescription drugs costing $109 million. Indirect costs of disease (eg, lost workdays) account for another $51 million.1 Since SD frequently manifests on the face, it tends to have negative effects on the patient’s quality of life, resulting in psychological distress and low self-esteem.2
Patients with SD may describe symptoms of excessive dandruff and itching along with hyperpigmentation or hypopigmentation of the skin; Black patients tend to present with the classic manifestations: a combination of scaling, flaking, and erythematous patches on the scalp, ears, and face, particularly around the eyebrows, eyelids, and nose. With SD being the second most common diagnosis in Black patients who seek care from a dermatologist, it is important to have effective treatment approaches for SD in this patient population.3
In this study, we aimed to evaluate medical and nonmedical treatment options for SD in Black patients by identifying common practices and products mentioned on consumer websites and in the medical literature.
Methods
A Google search was conducted during 2 time periods (September 2022—October 2022 and March 2023—April 2023) using the terms products for itchy scalp in Black patients, products for dandruff in Black patients, itchy scalp in Black women, itchy scalp in Black men, treatment for scalp itch in Black patients, and dry scalp in Black hair. Products that were recommended by at least 1 website on the first page of search results were included in our list of products, and the ingredients were reviewed by the authors. We excluded individual retailer websites as well as those that did not provide specific recommendations on products or ingredients to use when treating SD. To ensure reliability and standardization, we did not review products that were suggested by ads in the shopping section on the first page of search results.
We also evaluated medical treatments used for SD in dermatology literature. A PubMed search of articles indexed for MEDLINE using the terms seborrheic dermatitis treatment for Black patients, treatment for dandruff for Black patients, and seborrheic dermatitis and skin of color was conducted. We excluded articles that did not address treatment options for SD, were specific to treating SD in patient populations with specific comorbidities being studied, discussed SD in animals, or were published prior to 1990.
Results
We identified 16 unique consumer websites with product or ingredient recommendations for SD in Black patients, none of which were provided by authors with a medical or scientific background; however, 4 (25%) websites included insights from board-certified dermatologists. A total of 16 ingredients were recommended, 15 (94%) of which were mentioned at least twice in our search results (eTable 1).
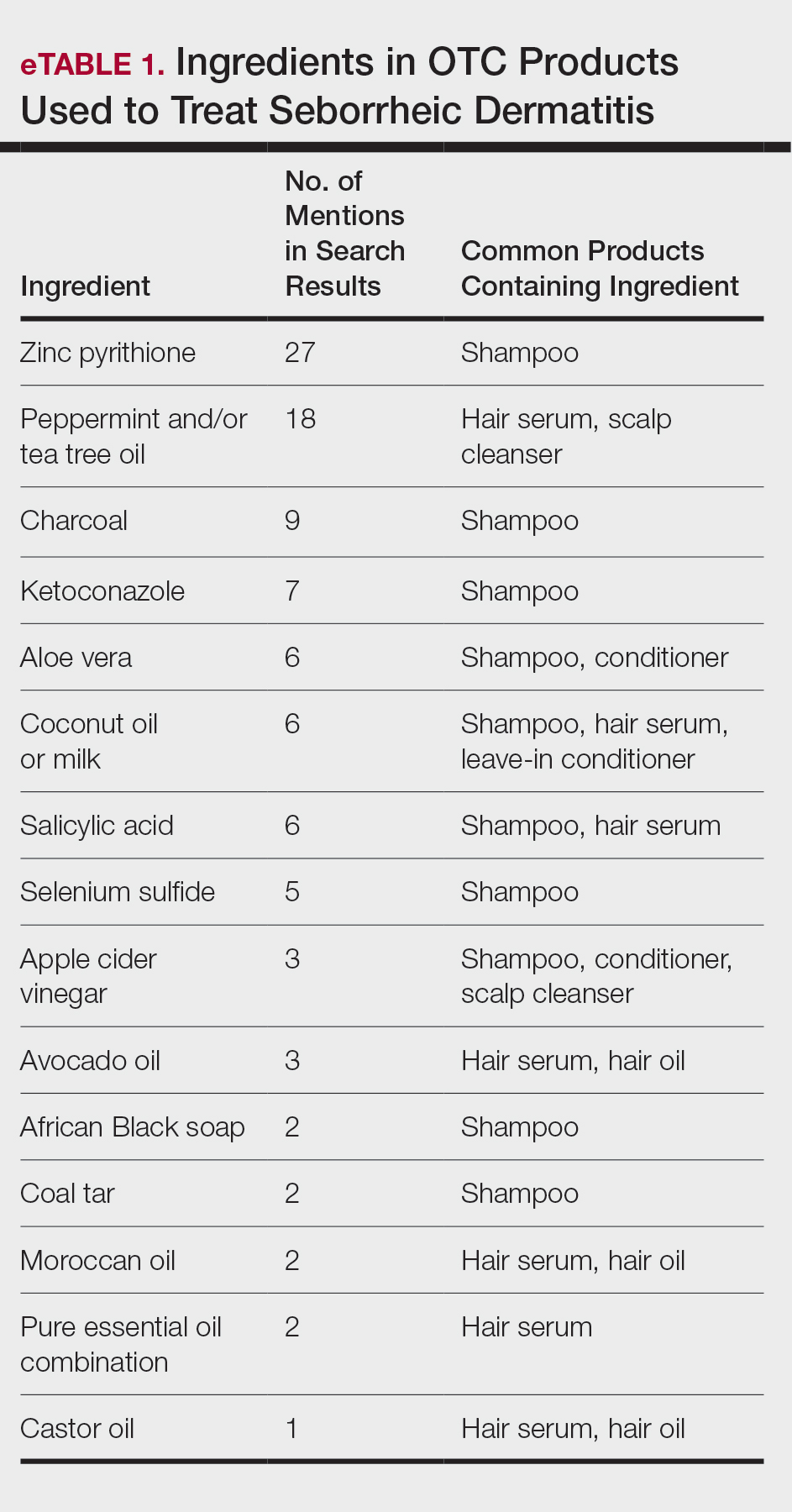
Overall, we noticed that ingredients labeled as natural or organic were common in over-the-counter (OTC) products, and ingredients such as sulfates and parabens were avoided. Common OTC ingredients for antidandruff and anti-itch shampoos and conditioners include zinc pyrithione, selenium sulfide, coal tar, salicylic acid, and citric acid. Additionally, coconut oil, tea tree oil, apple cider vinegar, and charcoal are common natural alternatives used to address SD symptoms.
Our review of the literature yielded limited recommendations tailored specifically to Black patients with SD. Of 108 abstracts, articles, or textbook chapters providing treatment recommendations for SD, 6 (6%) specifically discussed treatments for Black patients. All articles were written by authors with medical or scientific backgrounds. Of the treatment options discussed, topical antifungals generally were considered first-line for SD in all patients, with ketoconazole shampoo being a common first choice.4,5
Comment
Our study indicated that many consumer websites recommend unstudied nonmedical treatments for SD. Zinc pyrithione was one of the most commonly mentioned ingredients in OTC products to treat SD targeted toward Black patients, as its properties have contributed to ease of hair combing and less frizz.6 Zinc pyrithione has antifungal properties that reduce the proliferation of Malassezia furfur as well as anti-inflammatory properties that reduce irritation, pruritus, and erythema in areas affected by SD.7 Tea tree and peppermint oils also were commonly mentioned; the theory is that these oils mitigate SD by reducing yeast growth and soothing inflammation through antioxidant activity.8,9 Coal tar also is used due to its keratoplastic properties, which slow the growth of skin cells and ultimately reduce scaling and dryness.10 Yeast thrives in basic pH conditions; apple cider vinegar is used as an ingredient in OTC products for SD because its acidic pH creates a less favorable environment for yeast to grow.11 Although many of the ingredients found in OTC products we identified have not yet been studied, they have properties that theoretically would be helpful in treating SD.
Our review of the medical literature revealed that while there are treatments that are effective for SD, the recommended use may not consider the cultural differences that exist for Black patients. For instance, reports in the literature regarding ketoconazole shampoo revealed that ketoconazole increases the risk for hair shaft dryness, damage, and subsequent breakage, especially in Black women who also may be using heat styling or chemical relaxers.5 As a result, ketoconazole should be used with caution in Black women, with an emphasis on direct application to the scalp rather than the hair shafts.12 Additional options reported for Black patients include ciclopirox olamine and zinc pyrithione, which may have fewer risks.13
When prescribing medicated shampoos, traditional instructions regarding frequency of use to control symptoms of SD range from 2 to 3 times weekly to daily for a specified period of time determined by the dermatologist.14 However, frequency of hair washing varies greatly among Black patients, sometimes occurring only once monthly. The frequency also may change based on styling techniques (eg, braids, weaves, and wigs).15 Based on previous research underscoring the tendency for Black patients to use medicated shampoos less frequently than White patients, it is important for clinicians to understand that these cultural practices can undermine the effectiveness when medicated shampoos are prescribed for SD.16
Additionally, topical corticosteroids often are used in conjunction with antifungals to help decrease inflammation of the scalp.17 An option reported for Black patients is topical fluocinolone 0.01%; however, package instructions state to apply topically to the scalp nightly and wash the hair thoroughly each morning, which may not be feasible for Black patients based on previously mentioned differences in hair-washing techniques. An alternative option may be to apply the medication 3 to 4 times per week, washing the hair weekly rather than daily.18 Fluocinolone can be used as an ointment, solution, oil, or cream.19,20 When comparing treatment vehicles for SD, a study conducted by Chappell et al21 found that Black patients preferred using ointment or oil vehicles; White patients preferred foams and sprays, which may not be suitable for Afro hair patterns. As such, using less-drying modalities may increase compliance and treatment success in Black patients. For patients who may have involvement on the hairline, face, or ears along with hypopigmentation (which is a common skin concern associated with SD), calcineurin inhibitors can be used until resolution occurs.5,22 High et al15 found that twice-daily use of pimecrolimus rapidly normalized skin pigmentation during the first 2 weeks of use. Overall, personalization of treatment may not only avoid adverse effects but also ensure patient compliance, with the overall goal of treating to reduce yeast activity, pruritus, and dyschromia.22
Interestingly, after the website searches were completed for this study, the US Food and Drug Administration approved topical roflumilast foam for SD. In a phase III trial of 457 total patients, 36 Black patients were included.23 It was determined that 79.5% of patients overall throughout the trial achieved Investigator Global Assessment success (score of 0 [clear] or 1 [almost clear]) plus ≥2-point improvement from baseline (on a scale of 0 [clear] to 4 [severe]) at weeks 2, 4, and 8. Although there currently are no long-term studies, roflumilast may be a promising option for Black patients with SD.23
Aside from developing an individualized treatment approach for Black patients with SD, it is important to ask targeted questions during the clinical encounter to identify factors that may be exacerbating symptoms, especially due to the wide range of hair care practices used by the Black community (eTable 2). Asking targeted questions is especially important, as prior studies have shown that extensions, hair relaxers, and particular hair products can irritate the scalp and increase the likelihood of developing SD.21,24 Rucker Wright et al25 evaluated different hair care practices among young Black females and their association with the development of SD. The authors found that using hair extensions (either braided, cornrowed, or ponytails), chemical relaxers, and hair oils every 2 weeks was associated with SD. The study also found that SD rates were roughly 20% higher among Black girls with extensions compared to Black girls without extensions, regardless of how frequently hair was washed.25

Many Black patients grease the scalp with oils that are beneficial for lubrication and reduction of abrasive damage caused by grooming; however, they also may increase incidence of SD.26 Tight curls worn by Black patients also can impede sebum from traveling down the hair shaft, leading to oil buildup on the scalp. This is the ideal environment for increased Malassezia density and higher risk for SD development.27 To balance the beneficial effects of hair oils with the increased susceptibility for SD, providers should emphasize applying these oils only to distal hair shafts, which are more likely to be damaged, and avoiding application to the scalp.19
Conclusion
Given its long-term relapsing and remitting nature, SD can be distressing for Black patients, many of whom may seek additional treatment options aside from those recommended by health care professionals. In order to better educate patients, it is important for dermatologists to know not only the common ingredients that may be present in OTC products but also the thought process behind why patients use them. Additionally, prescription treatments for Black patients with SD may require nuanced alterations to the product instructions that may prevent health disparities and provide culturally sensitive care. Overall, the literature regarding treatment for Black patients with SD is limited, and more high-quality studies are needed.
- Tucker D, Masood S. Seborrheic dermatitis. StatPearls [Internet]. Updated March 1, 2024. Accessed December 19, 2024. https://www.ncbi.nlm.nih.gov/books/NBK551707/
- Borda LJ, Wikramanayake TC. Seborrheic dermatitis and dandruff: a comprehensive review. J Clin Investig Dermatol. 2015;3:10.13188 /2373-1044.1000019.
- American Academy of Dermatology. Seborrheic dermatitis by the numbers. American Academy of Dermatology Skin Disease Briefs. Updated May 5, 2018. Accessed November 22, 2024. https://www.aad.org/asset/49w949DPcF8RSJYIRHfDon
- Davis SA, Naarahari S, Feldman SR, et al. Top dermatologic conditions in patients of color: an analysis of nationally representative data. J Drugs Dermatol. 2012;11:466-473.
- Borda LJ, Perper M, Keri JE. Treatment of seborrheic dermatitis: a comprehensive review. J Dermatolog Treat. 2019;30:158-169.
- Draelos ZD, Kenneally DC, Hodges LT, et al. A comparison of hair quality and cosmetic acceptance following the use of two anti-dandruff shampoos. J Investig Dermatol Symp Proc. 2005;10:201-214.
- Barak-Shinar D, Green LJ. Scalp seborrheic dermatitis and dandruff therapy using a herbal and zinc pyrithione-based therapy of shampoo and scalp lotion. J Clin Aesthet Dermatol. 2018;11:26-31.
- Satchell AC, Saurajen A, Bell C, et al. Treatment of dandruff with 5% tea tree oil shampoo. J Am Acad Dermatol. 2002;47:852-855.
- Herro E, Jacob SE. Mentha piperita (peppermint). Dermatitis. 2010;21:327-329.
- Sanfilippo A, English JC. An overview of medicated shampoos used in dandruff treatment. Pharm Ther. 2006;31:396-400.
- Arun PVPS, Vineetha Y, Waheed M, et al. Quantification of the minimum amount of lemon juice and apple cider vinegar required for the growth inhibition of dandruff causing fungi Malassezia furfur. Int J Sci Res in Biological Sciences. 2019;6:144-147.
- Gao HY, Li Wan Po A. Topical formulations of fluocinolone acetonide. Are creams, gels and ointments bioequivalent and does dilution affect activity? Eur J Clin Pharmacol. 1994;46:71-75.
- Pauporte M, Maibach H, Lowe N, et al. Fluocinolone acetonide topical oil for scalp psoriasis. J Dermatolog Treat. 2004;15:360-364.
- Elgash M, Dlova N, Ogunleye T, et al. Seborrheic dermatitis in skin of color: clinical considerations. J Drugs Dermatol. 2019;18:24-27.
- High WA, Pandya AG. Pilot trial of 1% pimecrolimus cream in the treatment of seborrheic dermatitis in African American adults with associated hypopigmentation. J Am Acad Dermatol. 2006;54:1083-1088.
- Hollins LC, Butt M, Hong J, et al. Research in brief: survey of hair care practices in various ethnic and racial pediatric populations. Pediatr Dermatol. 2022;39:494-496.
- Halder RM, Roberts CI, Nootheti PK. Cutaneous diseases in the black races. Dermatol Clin. 2003;21:679-687, ix.
- Alexis AF, Sergay AB, Taylor SC. Common dermatologic disorders in skin of color: a comparative practice survey. Cutis. 2007;80:387-394.
- Friedmann DP, Mishra V, Batty T. Progressive facial papules in an African- American patient: an atypical presentation of seborrheic dermatitis. J Clin Aesthet Dermatol. 2018;11:44-45.
- Clark GW, Pope SM, Jaboori KA. Diagnosis and treatment of seborrheic dermatitis. Am Fam Physician. 2015;91:185-190.
- Chappell J, Mattox A, Simonetta C, et al. Seborrheic dermatitis of the scalp in populations practicing less frequent hair washing: ketoconazole 2% foam versus ketoconazole 2% shampoo. three-year data. J Am Acad Dermatol. 2014;70:AB54.
- Dadzie OE, Salam A. The hair grooming practices of women of African descent in London, United Kingdom: findings of a cross-sectional study. J Eur Acad Dermatol Venereol. 2016;30:1021-1024.
- Blauvelt A, Draelos ZD, Stein Gold L, et al. Roflumilast foam 0.3% for adolescent and adult patients with seborrheic dermatitis: a randomized, double-blinded, vehicle-controlled, phase 3 trial. J Am Acad Dermatol. 2024;90:986-993.
- Taylor SC, Barbosa V, Burgess C, et al. Hair and scalp disorders in adult and pediatric patients with skin of color. Cutis. 2017;100:31-35.
- Rucker Wright D, Gathers R, Kapke A, et al. Hair care practices and their association with scalp and hair disorders in African American girls. J Am Acad Dermatol. 2011;64:253-262.
- Raffi J, Suresh R, Agbai O. Clinical recognition and management of alopecia in women of color. Int J Womens Dermatol. 2019;5:314-319.
- Mayo T, Dinkins J, Elewski B. Hair oils may worsen seborrheic dermatitis in Black patients. Skin Appendage Disord. 2023;9:151-152.
Seborrheic dermatitis (SD) is a common chronic inflammatory skin condition that predominantly affects areas with high concentrations of sebaceous glands such as the scalp and face. Up to 5% of the worldwide population is affected by SD each year, causing a major burden of disease for patients and the health care system.1 In 2023, the cost of medical treatment for SD in the United States was $300 million, with outpatient office visits alone costing $58 million and prescription drugs costing $109 million. Indirect costs of disease (eg, lost workdays) account for another $51 million.1 Since SD frequently manifests on the face, it tends to have negative effects on the patient’s quality of life, resulting in psychological distress and low self-esteem.2
Patients with SD may describe symptoms of excessive dandruff and itching along with hyperpigmentation or hypopigmentation of the skin; Black patients tend to present with the classic manifestations: a combination of scaling, flaking, and erythematous patches on the scalp, ears, and face, particularly around the eyebrows, eyelids, and nose. With SD being the second most common diagnosis in Black patients who seek care from a dermatologist, it is important to have effective treatment approaches for SD in this patient population.3
In this study, we aimed to evaluate medical and nonmedical treatment options for SD in Black patients by identifying common practices and products mentioned on consumer websites and in the medical literature.
Methods
A Google search was conducted during 2 time periods (September 2022—October 2022 and March 2023—April 2023) using the terms products for itchy scalp in Black patients, products for dandruff in Black patients, itchy scalp in Black women, itchy scalp in Black men, treatment for scalp itch in Black patients, and dry scalp in Black hair. Products that were recommended by at least 1 website on the first page of search results were included in our list of products, and the ingredients were reviewed by the authors. We excluded individual retailer websites as well as those that did not provide specific recommendations on products or ingredients to use when treating SD. To ensure reliability and standardization, we did not review products that were suggested by ads in the shopping section on the first page of search results.
We also evaluated medical treatments used for SD in dermatology literature. A PubMed search of articles indexed for MEDLINE using the terms seborrheic dermatitis treatment for Black patients, treatment for dandruff for Black patients, and seborrheic dermatitis and skin of color was conducted. We excluded articles that did not address treatment options for SD, were specific to treating SD in patient populations with specific comorbidities being studied, discussed SD in animals, or were published prior to 1990.
Results
We identified 16 unique consumer websites with product or ingredient recommendations for SD in Black patients, none of which were provided by authors with a medical or scientific background; however, 4 (25%) websites included insights from board-certified dermatologists. A total of 16 ingredients were recommended, 15 (94%) of which were mentioned at least twice in our search results (eTable 1).

Overall, we noticed that ingredients labeled as natural or organic were common in over-the-counter (OTC) products, and ingredients such as sulfates and parabens were avoided. Common OTC ingredients for antidandruff and anti-itch shampoos and conditioners include zinc pyrithione, selenium sulfide, coal tar, salicylic acid, and citric acid. Additionally, coconut oil, tea tree oil, apple cider vinegar, and charcoal are common natural alternatives used to address SD symptoms.
Our review of the literature yielded limited recommendations tailored specifically to Black patients with SD. Of 108 abstracts, articles, or textbook chapters providing treatment recommendations for SD, 6 (6%) specifically discussed treatments for Black patients. All articles were written by authors with medical or scientific backgrounds. Of the treatment options discussed, topical antifungals generally were considered first-line for SD in all patients, with ketoconazole shampoo being a common first choice.4,5
Comment
Our study indicated that many consumer websites recommend unstudied nonmedical treatments for SD. Zinc pyrithione was one of the most commonly mentioned ingredients in OTC products to treat SD targeted toward Black patients, as its properties have contributed to ease of hair combing and less frizz.6 Zinc pyrithione has antifungal properties that reduce the proliferation of Malassezia furfur as well as anti-inflammatory properties that reduce irritation, pruritus, and erythema in areas affected by SD.7 Tea tree and peppermint oils also were commonly mentioned; the theory is that these oils mitigate SD by reducing yeast growth and soothing inflammation through antioxidant activity.8,9 Coal tar also is used due to its keratoplastic properties, which slow the growth of skin cells and ultimately reduce scaling and dryness.10 Yeast thrives in basic pH conditions; apple cider vinegar is used as an ingredient in OTC products for SD because its acidic pH creates a less favorable environment for yeast to grow.11 Although many of the ingredients found in OTC products we identified have not yet been studied, they have properties that theoretically would be helpful in treating SD.
Our review of the medical literature revealed that while there are treatments that are effective for SD, the recommended use may not consider the cultural differences that exist for Black patients. For instance, reports in the literature regarding ketoconazole shampoo revealed that ketoconazole increases the risk for hair shaft dryness, damage, and subsequent breakage, especially in Black women who also may be using heat styling or chemical relaxers.5 As a result, ketoconazole should be used with caution in Black women, with an emphasis on direct application to the scalp rather than the hair shafts.12 Additional options reported for Black patients include ciclopirox olamine and zinc pyrithione, which may have fewer risks.13
When prescribing medicated shampoos, traditional instructions regarding frequency of use to control symptoms of SD range from 2 to 3 times weekly to daily for a specified period of time determined by the dermatologist.14 However, frequency of hair washing varies greatly among Black patients, sometimes occurring only once monthly. The frequency also may change based on styling techniques (eg, braids, weaves, and wigs).15 Based on previous research underscoring the tendency for Black patients to use medicated shampoos less frequently than White patients, it is important for clinicians to understand that these cultural practices can undermine the effectiveness when medicated shampoos are prescribed for SD.16
Additionally, topical corticosteroids often are used in conjunction with antifungals to help decrease inflammation of the scalp.17 An option reported for Black patients is topical fluocinolone 0.01%; however, package instructions state to apply topically to the scalp nightly and wash the hair thoroughly each morning, which may not be feasible for Black patients based on previously mentioned differences in hair-washing techniques. An alternative option may be to apply the medication 3 to 4 times per week, washing the hair weekly rather than daily.18 Fluocinolone can be used as an ointment, solution, oil, or cream.19,20 When comparing treatment vehicles for SD, a study conducted by Chappell et al21 found that Black patients preferred using ointment or oil vehicles; White patients preferred foams and sprays, which may not be suitable for Afro hair patterns. As such, using less-drying modalities may increase compliance and treatment success in Black patients. For patients who may have involvement on the hairline, face, or ears along with hypopigmentation (which is a common skin concern associated with SD), calcineurin inhibitors can be used until resolution occurs.5,22 High et al15 found that twice-daily use of pimecrolimus rapidly normalized skin pigmentation during the first 2 weeks of use. Overall, personalization of treatment may not only avoid adverse effects but also ensure patient compliance, with the overall goal of treating to reduce yeast activity, pruritus, and dyschromia.22
Interestingly, after the website searches were completed for this study, the US Food and Drug Administration approved topical roflumilast foam for SD. In a phase III trial of 457 total patients, 36 Black patients were included.23 It was determined that 79.5% of patients overall throughout the trial achieved Investigator Global Assessment success (score of 0 [clear] or 1 [almost clear]) plus ≥2-point improvement from baseline (on a scale of 0 [clear] to 4 [severe]) at weeks 2, 4, and 8. Although there currently are no long-term studies, roflumilast may be a promising option for Black patients with SD.23
Aside from developing an individualized treatment approach for Black patients with SD, it is important to ask targeted questions during the clinical encounter to identify factors that may be exacerbating symptoms, especially due to the wide range of hair care practices used by the Black community (eTable 2). Asking targeted questions is especially important, as prior studies have shown that extensions, hair relaxers, and particular hair products can irritate the scalp and increase the likelihood of developing SD.21,24 Rucker Wright et al25 evaluated different hair care practices among young Black females and their association with the development of SD. The authors found that using hair extensions (either braided, cornrowed, or ponytails), chemical relaxers, and hair oils every 2 weeks was associated with SD. The study also found that SD rates were roughly 20% higher among Black girls with extensions compared to Black girls without extensions, regardless of how frequently hair was washed.25

Many Black patients grease the scalp with oils that are beneficial for lubrication and reduction of abrasive damage caused by grooming; however, they also may increase incidence of SD.26 Tight curls worn by Black patients also can impede sebum from traveling down the hair shaft, leading to oil buildup on the scalp. This is the ideal environment for increased Malassezia density and higher risk for SD development.27 To balance the beneficial effects of hair oils with the increased susceptibility for SD, providers should emphasize applying these oils only to distal hair shafts, which are more likely to be damaged, and avoiding application to the scalp.19
Conclusion
Given its long-term relapsing and remitting nature, SD can be distressing for Black patients, many of whom may seek additional treatment options aside from those recommended by health care professionals. In order to better educate patients, it is important for dermatologists to know not only the common ingredients that may be present in OTC products but also the thought process behind why patients use them. Additionally, prescription treatments for Black patients with SD may require nuanced alterations to the product instructions that may prevent health disparities and provide culturally sensitive care. Overall, the literature regarding treatment for Black patients with SD is limited, and more high-quality studies are needed.
Seborrheic dermatitis (SD) is a common chronic inflammatory skin condition that predominantly affects areas with high concentrations of sebaceous glands such as the scalp and face. Up to 5% of the worldwide population is affected by SD each year, causing a major burden of disease for patients and the health care system.1 In 2023, the cost of medical treatment for SD in the United States was $300 million, with outpatient office visits alone costing $58 million and prescription drugs costing $109 million. Indirect costs of disease (eg, lost workdays) account for another $51 million.1 Since SD frequently manifests on the face, it tends to have negative effects on the patient’s quality of life, resulting in psychological distress and low self-esteem.2
Patients with SD may describe symptoms of excessive dandruff and itching along with hyperpigmentation or hypopigmentation of the skin; Black patients tend to present with the classic manifestations: a combination of scaling, flaking, and erythematous patches on the scalp, ears, and face, particularly around the eyebrows, eyelids, and nose. With SD being the second most common diagnosis in Black patients who seek care from a dermatologist, it is important to have effective treatment approaches for SD in this patient population.3
In this study, we aimed to evaluate medical and nonmedical treatment options for SD in Black patients by identifying common practices and products mentioned on consumer websites and in the medical literature.
Methods
A Google search was conducted during 2 time periods (September 2022—October 2022 and March 2023—April 2023) using the terms products for itchy scalp in Black patients, products for dandruff in Black patients, itchy scalp in Black women, itchy scalp in Black men, treatment for scalp itch in Black patients, and dry scalp in Black hair. Products that were recommended by at least 1 website on the first page of search results were included in our list of products, and the ingredients were reviewed by the authors. We excluded individual retailer websites as well as those that did not provide specific recommendations on products or ingredients to use when treating SD. To ensure reliability and standardization, we did not review products that were suggested by ads in the shopping section on the first page of search results.
We also evaluated medical treatments used for SD in dermatology literature. A PubMed search of articles indexed for MEDLINE using the terms seborrheic dermatitis treatment for Black patients, treatment for dandruff for Black patients, and seborrheic dermatitis and skin of color was conducted. We excluded articles that did not address treatment options for SD, were specific to treating SD in patient populations with specific comorbidities being studied, discussed SD in animals, or were published prior to 1990.
Results
We identified 16 unique consumer websites with product or ingredient recommendations for SD in Black patients, none of which were provided by authors with a medical or scientific background; however, 4 (25%) websites included insights from board-certified dermatologists. A total of 16 ingredients were recommended, 15 (94%) of which were mentioned at least twice in our search results (eTable 1).

Overall, we noticed that ingredients labeled as natural or organic were common in over-the-counter (OTC) products, and ingredients such as sulfates and parabens were avoided. Common OTC ingredients for antidandruff and anti-itch shampoos and conditioners include zinc pyrithione, selenium sulfide, coal tar, salicylic acid, and citric acid. Additionally, coconut oil, tea tree oil, apple cider vinegar, and charcoal are common natural alternatives used to address SD symptoms.
Our review of the literature yielded limited recommendations tailored specifically to Black patients with SD. Of 108 abstracts, articles, or textbook chapters providing treatment recommendations for SD, 6 (6%) specifically discussed treatments for Black patients. All articles were written by authors with medical or scientific backgrounds. Of the treatment options discussed, topical antifungals generally were considered first-line for SD in all patients, with ketoconazole shampoo being a common first choice.4,5
Comment
Our study indicated that many consumer websites recommend unstudied nonmedical treatments for SD. Zinc pyrithione was one of the most commonly mentioned ingredients in OTC products to treat SD targeted toward Black patients, as its properties have contributed to ease of hair combing and less frizz.6 Zinc pyrithione has antifungal properties that reduce the proliferation of Malassezia furfur as well as anti-inflammatory properties that reduce irritation, pruritus, and erythema in areas affected by SD.7 Tea tree and peppermint oils also were commonly mentioned; the theory is that these oils mitigate SD by reducing yeast growth and soothing inflammation through antioxidant activity.8,9 Coal tar also is used due to its keratoplastic properties, which slow the growth of skin cells and ultimately reduce scaling and dryness.10 Yeast thrives in basic pH conditions; apple cider vinegar is used as an ingredient in OTC products for SD because its acidic pH creates a less favorable environment for yeast to grow.11 Although many of the ingredients found in OTC products we identified have not yet been studied, they have properties that theoretically would be helpful in treating SD.
Our review of the medical literature revealed that while there are treatments that are effective for SD, the recommended use may not consider the cultural differences that exist for Black patients. For instance, reports in the literature regarding ketoconazole shampoo revealed that ketoconazole increases the risk for hair shaft dryness, damage, and subsequent breakage, especially in Black women who also may be using heat styling or chemical relaxers.5 As a result, ketoconazole should be used with caution in Black women, with an emphasis on direct application to the scalp rather than the hair shafts.12 Additional options reported for Black patients include ciclopirox olamine and zinc pyrithione, which may have fewer risks.13
When prescribing medicated shampoos, traditional instructions regarding frequency of use to control symptoms of SD range from 2 to 3 times weekly to daily for a specified period of time determined by the dermatologist.14 However, frequency of hair washing varies greatly among Black patients, sometimes occurring only once monthly. The frequency also may change based on styling techniques (eg, braids, weaves, and wigs).15 Based on previous research underscoring the tendency for Black patients to use medicated shampoos less frequently than White patients, it is important for clinicians to understand that these cultural practices can undermine the effectiveness when medicated shampoos are prescribed for SD.16
Additionally, topical corticosteroids often are used in conjunction with antifungals to help decrease inflammation of the scalp.17 An option reported for Black patients is topical fluocinolone 0.01%; however, package instructions state to apply topically to the scalp nightly and wash the hair thoroughly each morning, which may not be feasible for Black patients based on previously mentioned differences in hair-washing techniques. An alternative option may be to apply the medication 3 to 4 times per week, washing the hair weekly rather than daily.18 Fluocinolone can be used as an ointment, solution, oil, or cream.19,20 When comparing treatment vehicles for SD, a study conducted by Chappell et al21 found that Black patients preferred using ointment or oil vehicles; White patients preferred foams and sprays, which may not be suitable for Afro hair patterns. As such, using less-drying modalities may increase compliance and treatment success in Black patients. For patients who may have involvement on the hairline, face, or ears along with hypopigmentation (which is a common skin concern associated with SD), calcineurin inhibitors can be used until resolution occurs.5,22 High et al15 found that twice-daily use of pimecrolimus rapidly normalized skin pigmentation during the first 2 weeks of use. Overall, personalization of treatment may not only avoid adverse effects but also ensure patient compliance, with the overall goal of treating to reduce yeast activity, pruritus, and dyschromia.22
Interestingly, after the website searches were completed for this study, the US Food and Drug Administration approved topical roflumilast foam for SD. In a phase III trial of 457 total patients, 36 Black patients were included.23 It was determined that 79.5% of patients overall throughout the trial achieved Investigator Global Assessment success (score of 0 [clear] or 1 [almost clear]) plus ≥2-point improvement from baseline (on a scale of 0 [clear] to 4 [severe]) at weeks 2, 4, and 8. Although there currently are no long-term studies, roflumilast may be a promising option for Black patients with SD.23
Aside from developing an individualized treatment approach for Black patients with SD, it is important to ask targeted questions during the clinical encounter to identify factors that may be exacerbating symptoms, especially due to the wide range of hair care practices used by the Black community (eTable 2). Asking targeted questions is especially important, as prior studies have shown that extensions, hair relaxers, and particular hair products can irritate the scalp and increase the likelihood of developing SD.21,24 Rucker Wright et al25 evaluated different hair care practices among young Black females and their association with the development of SD. The authors found that using hair extensions (either braided, cornrowed, or ponytails), chemical relaxers, and hair oils every 2 weeks was associated with SD. The study also found that SD rates were roughly 20% higher among Black girls with extensions compared to Black girls without extensions, regardless of how frequently hair was washed.25

Many Black patients grease the scalp with oils that are beneficial for lubrication and reduction of abrasive damage caused by grooming; however, they also may increase incidence of SD.26 Tight curls worn by Black patients also can impede sebum from traveling down the hair shaft, leading to oil buildup on the scalp. This is the ideal environment for increased Malassezia density and higher risk for SD development.27 To balance the beneficial effects of hair oils with the increased susceptibility for SD, providers should emphasize applying these oils only to distal hair shafts, which are more likely to be damaged, and avoiding application to the scalp.19
Conclusion
Given its long-term relapsing and remitting nature, SD can be distressing for Black patients, many of whom may seek additional treatment options aside from those recommended by health care professionals. In order to better educate patients, it is important for dermatologists to know not only the common ingredients that may be present in OTC products but also the thought process behind why patients use them. Additionally, prescription treatments for Black patients with SD may require nuanced alterations to the product instructions that may prevent health disparities and provide culturally sensitive care. Overall, the literature regarding treatment for Black patients with SD is limited, and more high-quality studies are needed.
- Tucker D, Masood S. Seborrheic dermatitis. StatPearls [Internet]. Updated March 1, 2024. Accessed December 19, 2024. https://www.ncbi.nlm.nih.gov/books/NBK551707/
- Borda LJ, Wikramanayake TC. Seborrheic dermatitis and dandruff: a comprehensive review. J Clin Investig Dermatol. 2015;3:10.13188 /2373-1044.1000019.
- American Academy of Dermatology. Seborrheic dermatitis by the numbers. American Academy of Dermatology Skin Disease Briefs. Updated May 5, 2018. Accessed November 22, 2024. https://www.aad.org/asset/49w949DPcF8RSJYIRHfDon
- Davis SA, Naarahari S, Feldman SR, et al. Top dermatologic conditions in patients of color: an analysis of nationally representative data. J Drugs Dermatol. 2012;11:466-473.
- Borda LJ, Perper M, Keri JE. Treatment of seborrheic dermatitis: a comprehensive review. J Dermatolog Treat. 2019;30:158-169.
- Draelos ZD, Kenneally DC, Hodges LT, et al. A comparison of hair quality and cosmetic acceptance following the use of two anti-dandruff shampoos. J Investig Dermatol Symp Proc. 2005;10:201-214.
- Barak-Shinar D, Green LJ. Scalp seborrheic dermatitis and dandruff therapy using a herbal and zinc pyrithione-based therapy of shampoo and scalp lotion. J Clin Aesthet Dermatol. 2018;11:26-31.
- Satchell AC, Saurajen A, Bell C, et al. Treatment of dandruff with 5% tea tree oil shampoo. J Am Acad Dermatol. 2002;47:852-855.
- Herro E, Jacob SE. Mentha piperita (peppermint). Dermatitis. 2010;21:327-329.
- Sanfilippo A, English JC. An overview of medicated shampoos used in dandruff treatment. Pharm Ther. 2006;31:396-400.
- Arun PVPS, Vineetha Y, Waheed M, et al. Quantification of the minimum amount of lemon juice and apple cider vinegar required for the growth inhibition of dandruff causing fungi Malassezia furfur. Int J Sci Res in Biological Sciences. 2019;6:144-147.
- Gao HY, Li Wan Po A. Topical formulations of fluocinolone acetonide. Are creams, gels and ointments bioequivalent and does dilution affect activity? Eur J Clin Pharmacol. 1994;46:71-75.
- Pauporte M, Maibach H, Lowe N, et al. Fluocinolone acetonide topical oil for scalp psoriasis. J Dermatolog Treat. 2004;15:360-364.
- Elgash M, Dlova N, Ogunleye T, et al. Seborrheic dermatitis in skin of color: clinical considerations. J Drugs Dermatol. 2019;18:24-27.
- High WA, Pandya AG. Pilot trial of 1% pimecrolimus cream in the treatment of seborrheic dermatitis in African American adults with associated hypopigmentation. J Am Acad Dermatol. 2006;54:1083-1088.
- Hollins LC, Butt M, Hong J, et al. Research in brief: survey of hair care practices in various ethnic and racial pediatric populations. Pediatr Dermatol. 2022;39:494-496.
- Halder RM, Roberts CI, Nootheti PK. Cutaneous diseases in the black races. Dermatol Clin. 2003;21:679-687, ix.
- Alexis AF, Sergay AB, Taylor SC. Common dermatologic disorders in skin of color: a comparative practice survey. Cutis. 2007;80:387-394.
- Friedmann DP, Mishra V, Batty T. Progressive facial papules in an African- American patient: an atypical presentation of seborrheic dermatitis. J Clin Aesthet Dermatol. 2018;11:44-45.
- Clark GW, Pope SM, Jaboori KA. Diagnosis and treatment of seborrheic dermatitis. Am Fam Physician. 2015;91:185-190.
- Chappell J, Mattox A, Simonetta C, et al. Seborrheic dermatitis of the scalp in populations practicing less frequent hair washing: ketoconazole 2% foam versus ketoconazole 2% shampoo. three-year data. J Am Acad Dermatol. 2014;70:AB54.
- Dadzie OE, Salam A. The hair grooming practices of women of African descent in London, United Kingdom: findings of a cross-sectional study. J Eur Acad Dermatol Venereol. 2016;30:1021-1024.
- Blauvelt A, Draelos ZD, Stein Gold L, et al. Roflumilast foam 0.3% for adolescent and adult patients with seborrheic dermatitis: a randomized, double-blinded, vehicle-controlled, phase 3 trial. J Am Acad Dermatol. 2024;90:986-993.
- Taylor SC, Barbosa V, Burgess C, et al. Hair and scalp disorders in adult and pediatric patients with skin of color. Cutis. 2017;100:31-35.
- Rucker Wright D, Gathers R, Kapke A, et al. Hair care practices and their association with scalp and hair disorders in African American girls. J Am Acad Dermatol. 2011;64:253-262.
- Raffi J, Suresh R, Agbai O. Clinical recognition and management of alopecia in women of color. Int J Womens Dermatol. 2019;5:314-319.
- Mayo T, Dinkins J, Elewski B. Hair oils may worsen seborrheic dermatitis in Black patients. Skin Appendage Disord. 2023;9:151-152.
- Tucker D, Masood S. Seborrheic dermatitis. StatPearls [Internet]. Updated March 1, 2024. Accessed December 19, 2024. https://www.ncbi.nlm.nih.gov/books/NBK551707/
- Borda LJ, Wikramanayake TC. Seborrheic dermatitis and dandruff: a comprehensive review. J Clin Investig Dermatol. 2015;3:10.13188 /2373-1044.1000019.
- American Academy of Dermatology. Seborrheic dermatitis by the numbers. American Academy of Dermatology Skin Disease Briefs. Updated May 5, 2018. Accessed November 22, 2024. https://www.aad.org/asset/49w949DPcF8RSJYIRHfDon
- Davis SA, Naarahari S, Feldman SR, et al. Top dermatologic conditions in patients of color: an analysis of nationally representative data. J Drugs Dermatol. 2012;11:466-473.
- Borda LJ, Perper M, Keri JE. Treatment of seborrheic dermatitis: a comprehensive review. J Dermatolog Treat. 2019;30:158-169.
- Draelos ZD, Kenneally DC, Hodges LT, et al. A comparison of hair quality and cosmetic acceptance following the use of two anti-dandruff shampoos. J Investig Dermatol Symp Proc. 2005;10:201-214.
- Barak-Shinar D, Green LJ. Scalp seborrheic dermatitis and dandruff therapy using a herbal and zinc pyrithione-based therapy of shampoo and scalp lotion. J Clin Aesthet Dermatol. 2018;11:26-31.
- Satchell AC, Saurajen A, Bell C, et al. Treatment of dandruff with 5% tea tree oil shampoo. J Am Acad Dermatol. 2002;47:852-855.
- Herro E, Jacob SE. Mentha piperita (peppermint). Dermatitis. 2010;21:327-329.
- Sanfilippo A, English JC. An overview of medicated shampoos used in dandruff treatment. Pharm Ther. 2006;31:396-400.
- Arun PVPS, Vineetha Y, Waheed M, et al. Quantification of the minimum amount of lemon juice and apple cider vinegar required for the growth inhibition of dandruff causing fungi Malassezia furfur. Int J Sci Res in Biological Sciences. 2019;6:144-147.
- Gao HY, Li Wan Po A. Topical formulations of fluocinolone acetonide. Are creams, gels and ointments bioequivalent and does dilution affect activity? Eur J Clin Pharmacol. 1994;46:71-75.
- Pauporte M, Maibach H, Lowe N, et al. Fluocinolone acetonide topical oil for scalp psoriasis. J Dermatolog Treat. 2004;15:360-364.
- Elgash M, Dlova N, Ogunleye T, et al. Seborrheic dermatitis in skin of color: clinical considerations. J Drugs Dermatol. 2019;18:24-27.
- High WA, Pandya AG. Pilot trial of 1% pimecrolimus cream in the treatment of seborrheic dermatitis in African American adults with associated hypopigmentation. J Am Acad Dermatol. 2006;54:1083-1088.
- Hollins LC, Butt M, Hong J, et al. Research in brief: survey of hair care practices in various ethnic and racial pediatric populations. Pediatr Dermatol. 2022;39:494-496.
- Halder RM, Roberts CI, Nootheti PK. Cutaneous diseases in the black races. Dermatol Clin. 2003;21:679-687, ix.
- Alexis AF, Sergay AB, Taylor SC. Common dermatologic disorders in skin of color: a comparative practice survey. Cutis. 2007;80:387-394.
- Friedmann DP, Mishra V, Batty T. Progressive facial papules in an African- American patient: an atypical presentation of seborrheic dermatitis. J Clin Aesthet Dermatol. 2018;11:44-45.
- Clark GW, Pope SM, Jaboori KA. Diagnosis and treatment of seborrheic dermatitis. Am Fam Physician. 2015;91:185-190.
- Chappell J, Mattox A, Simonetta C, et al. Seborrheic dermatitis of the scalp in populations practicing less frequent hair washing: ketoconazole 2% foam versus ketoconazole 2% shampoo. three-year data. J Am Acad Dermatol. 2014;70:AB54.
- Dadzie OE, Salam A. The hair grooming practices of women of African descent in London, United Kingdom: findings of a cross-sectional study. J Eur Acad Dermatol Venereol. 2016;30:1021-1024.
- Blauvelt A, Draelos ZD, Stein Gold L, et al. Roflumilast foam 0.3% for adolescent and adult patients with seborrheic dermatitis: a randomized, double-blinded, vehicle-controlled, phase 3 trial. J Am Acad Dermatol. 2024;90:986-993.
- Taylor SC, Barbosa V, Burgess C, et al. Hair and scalp disorders in adult and pediatric patients with skin of color. Cutis. 2017;100:31-35.
- Rucker Wright D, Gathers R, Kapke A, et al. Hair care practices and their association with scalp and hair disorders in African American girls. J Am Acad Dermatol. 2011;64:253-262.
- Raffi J, Suresh R, Agbai O. Clinical recognition and management of alopecia in women of color. Int J Womens Dermatol. 2019;5:314-319.
- Mayo T, Dinkins J, Elewski B. Hair oils may worsen seborrheic dermatitis in Black patients. Skin Appendage Disord. 2023;9:151-152.
Treatment of Seborrheic Dermatitis in Black Patients
Treatment of Seborrheic Dermatitis in Black Patients
PRACTICE POINTS
- Cultural awareness when treating Black patients with seborrheic dermatitis is vital to providing appropriate care, as hair care practices may impact treatment options and regimen.
- Knowledge about over-the-counter products that are targeted toward Black patients and the ingredients they contain can assist in providing better counseling to patients and improve shared decision-making.
Cultural Respect vs Individual Patient Autonomy: A Delicate Balancing Act
Cultural competency is one of the most important values in the practice of medicine. Defined as the “ability to collaborate effectively with individuals from different cultures,” this type of competence “improves healthcare experiences and outcomes.” But within the context of cultural familiarity, it’s equally important to “understand that each person is an individual and may or may not adhere to certain cultural beliefs or practices common in his or her culture,” according to the Agency for Healthcare Research and Quality’s (AHRQ’s) Health Literacy Universal Precautions Toolkit.
Sarah Candler, MD, MPH, an internal medicine physician specializing in primary care for older adults in Washington, DC, said that the medical code of ethics consists of several pillars, with patient autonomy as the “first and most primary of those pillars.” She calls the balance of patient autonomy and cultural respect a “complicated tightrope to walk,” but says that these ethical principles can inform medical decisions and the patient-physician relationship.
Cultural Familiarity
It’s important to be as familiar as possible with the patient’s culture, Santina Wheat, MD, program director, Northwestern McGaw Family Medicine Residency at Delnor Hospital, Geneva, told this news organization. “For example, we serve many Orthodox Jewish patients. We had a meeting with rabbis from the community to present to us what religious laws might affect our patients. Until recently, I was delivering babies, and there was always a 24-hour emergency rabbi on call if an Orthodox patient wanted the input of a rabbi into her decisions.”
Jay W. Lee, MD, MPH, a member of the board of directors of the American Academy of Family Physicians, also sets out to educate himself about the cultural norms of his patients if they come from populations he’s not familiar with. “For example, this comes up when a new refugee population comes to the United States — most recently, there was a population of Afghan refugees,” Lee told this news organization.
Lee spent “a lot of time trying to learn about their cultural norms,” which prepared him to “ask more targeted questions about the patient’s understanding of the tests we were ordering or treatment options we were bringing forward.”
Lee, also the medical director at Integrated Health Partners of Southern California and associate clinical professor of family medicine at the University of California, Irvine, said it might be best if the physician is “language congruent or culturally similar.” Lee is of Asian descent and also speaks Spanish fluently. “I enjoy cultural exchanges with my patients, and I encourage patients to find a physician who’s the best fit.” But being from the same culture isn’t absolutely necessary for building relationships with the patient. “The key is offering the patient autonomy” while understanding the cultural context.
Don’t Assume ... Always Ask
Cultural familiarity doesn’t equate with stereotyping, Wheat emphasized. “Proceeding without assumptions opens the opportunity to ask questions for clarification and understanding and to improve patient care,” said Lee.
Sara Glass, PhD, LCSW, agrees. She’s the clinical director of Soul Wellness NYC, New York City, a psychotherapy practice that specializes in treating trauma. Based on her own experiences, she knows that some physicians and other healthcare professionals confuse cultural sensitivity with cultural stereotyping.
Glass, formerly Hasidic and ultra-Orthodox, shared an example from her own life. During the delivery of her second child, she sustained a vaginal tear. At her 6-week postpartum visit, her ob/gyn said, “Just remind me when you’re in your ninth month next time, and I can sew it up right after you deliver.”
Much of this physician’s practice “consisted of Hasidic women who looked just like me, wearing the same garb — head coverings such as wigs and scarves and long skirts. Most women in that community have multiple pregnancies,” Glass told this news organization. “My sister has 10 children, and that’s not unusual. The doctor simply assumed I’d be going on to have more babies without asking if that’s what I wanted.”
Glass says she was also never given information by her physician about the range of available contraceptive options. The rabbis of the Hasidic sect to which Glass belonged allowed women to practice contraception for 6 months following childbirth, or for longer, in the setting of certain medical conditions, but only certain types of birth control were religiously permissible. Other options were not mentioned to her by her physician, and she didn’t know that they existed.
Making no assumptions applies not only to patients from other cultures but also to all patients — including members of “mainstream American culture.”
Candler recalls a young patient with a new baby, who shared “how exhausted she was and how much time, energy, and work it took to care for children,” Candler recounted. “To me, it sounded as though she didn’t want another child, and I was about to offer contraception when it occurred to me to first ask if she wanted to have more children.” Candler was surprised when the patient said that, although she wasn’t actively looking to become pregnant again, she didn’t want to take preventive measures. “I’m so glad I asked, rather than simply assuming.”
Culture Is Mutable
Important questions to ask patients include whether there are aspects of their culture or religion that might affect their care — which can include medications they may feel uncomfortable using — and what family members they want to have involved in clinical discussions and decisions, said Wheat.
Lee described treating a refugee from Afghanistan who was in her sixth month of pregnancy. “I quickly needed to learn about what her expectations were for her care and my presence as a male on her care team,” he recounted. Lee arranged for the patient to receive prenatal care from a different clinician and arranged for follow-up for her husband and children. “Everyone had good results.”
Candler noted that some patients choose their physician specifically because that practitioner is conversant with their culture and respectful of its mores — especially when physicians share the same culture as the patient. But that level of familiarity can make it easy to forget to ask questions about the experience of the individual patient within that culture.
Moreover, Glass suggested, some physicians who treat patients from a particular culture or religious group may be concerned about offending them or antagonizing religious leaders if they discuss medical options that aren’t accepted or practiced in that community or culture, such as vasectomy for male contraception. “But that deprives patients of knowing what choices are available and making truly informed decisions.”
This is especially important because “culture is mutable,” said Candler, and religious or cultural practices can “look one way on paper but be implemented, adopted, or executed in a completely different way by every human being who lives in that culture.” The best cultural competency “comes from continuing to build relationships with our patients. But even in a single visit, a single hospitalization, we should get to know patients as human beings, not just members of a given culture.”
There are cultures in which families want to be the liaison between the patient and the physician and to make decisions on the patient’s behalf. “I always ask patients what role they want their family members to play even if the cultural expectation is that the family will be heavily involved,” Candler said.
Sometimes, this can be awkward, and families might become upset. Candler described an elderly, frail patient who was diagnosed with end-stage cancer. She had always relied heavily on family to care for her. Concerned about overburdening them, she didn’t want them to know her diagnosis. The patient was mentally competent to make that decision.
“Usually, I would have had the family at the bedside so I could be sure everyone was appropriately informed and prepared for what lay ahead, but in this case, I couldn’t do so,” Candler said. “I had to inform her entire care team not to discuss the cancer diagnosis with any family members because this was the patient’s express wish. And when the family asked me if the diagnosis was cancer, I had to respond, ‘I’m so sorry, but your loved one doesn’t want us to discuss details of her diagnosis.’”
Other patients don’t want to know their own diagnosis and specifically ask Candler to inform a family member. “I’ve had patients request that I tell their children. They want their children to make decisions on their behalf.”
The main thing, Candler emphasized, is to “ask the patient, make sure the patient is competent to make that decision, thoroughly document the patient’s decision in the chart, and respect whatever that decision is.”
You Can Revisit the Questions
Having a longitudinal relationship means that the physician can revisit the same questions at different junctures because people’s perspectives sometimes change over time. “Discussing what a patient wants isn’t necessarily a one-time occurrence,” Wheat said. For example, “I’ve had situations where a patient has been a member of Jehovah’s Witnesses and won’t accept blood products — like transfusions — in treatment. I tell these patients that if an emergent situation arises, I would like to have the conversation again.”
Of course, sometimes patients are seen in the emergency department or in other situations where the physician has no prior relationship with them. “I always go into a room, especially with new patients, aiming to build rapport, communicate with a high level of respect, introduce myself, explain my approach, and understand the patient’s wishes,” Lee said. “As scenarios play out, I ask in multiple ways for the patient to confirm those wishes.”
He acknowledges that this can be time-consuming, “but it helps ensure the care that patient receives is complete, thorough, comprehensive, and respectful of the patient’s values and wishes.”
Candler disclosed paid part-time clinical work at CuraCapitol Primary Care Services, volunteer advocacy (reimbursed for travel) for the American College of Physicians, volunteer advocacy (reimbursed for travel) for the American Medical Association while serving on their Task Force to Preserve the Patient-Physician Relationship, and serving as a partner representative (reimbursed for time) for the AHRQ’s Person-Centered Care Planning Partnership, representing the American College of Physicians. Lee, Wheat, and Glass disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Cultural competency is one of the most important values in the practice of medicine. Defined as the “ability to collaborate effectively with individuals from different cultures,” this type of competence “improves healthcare experiences and outcomes.” But within the context of cultural familiarity, it’s equally important to “understand that each person is an individual and may or may not adhere to certain cultural beliefs or practices common in his or her culture,” according to the Agency for Healthcare Research and Quality’s (AHRQ’s) Health Literacy Universal Precautions Toolkit.
Sarah Candler, MD, MPH, an internal medicine physician specializing in primary care for older adults in Washington, DC, said that the medical code of ethics consists of several pillars, with patient autonomy as the “first and most primary of those pillars.” She calls the balance of patient autonomy and cultural respect a “complicated tightrope to walk,” but says that these ethical principles can inform medical decisions and the patient-physician relationship.
Cultural Familiarity
It’s important to be as familiar as possible with the patient’s culture, Santina Wheat, MD, program director, Northwestern McGaw Family Medicine Residency at Delnor Hospital, Geneva, told this news organization. “For example, we serve many Orthodox Jewish patients. We had a meeting with rabbis from the community to present to us what religious laws might affect our patients. Until recently, I was delivering babies, and there was always a 24-hour emergency rabbi on call if an Orthodox patient wanted the input of a rabbi into her decisions.”
Jay W. Lee, MD, MPH, a member of the board of directors of the American Academy of Family Physicians, also sets out to educate himself about the cultural norms of his patients if they come from populations he’s not familiar with. “For example, this comes up when a new refugee population comes to the United States — most recently, there was a population of Afghan refugees,” Lee told this news organization.
Lee spent “a lot of time trying to learn about their cultural norms,” which prepared him to “ask more targeted questions about the patient’s understanding of the tests we were ordering or treatment options we were bringing forward.”
Lee, also the medical director at Integrated Health Partners of Southern California and associate clinical professor of family medicine at the University of California, Irvine, said it might be best if the physician is “language congruent or culturally similar.” Lee is of Asian descent and also speaks Spanish fluently. “I enjoy cultural exchanges with my patients, and I encourage patients to find a physician who’s the best fit.” But being from the same culture isn’t absolutely necessary for building relationships with the patient. “The key is offering the patient autonomy” while understanding the cultural context.
Don’t Assume ... Always Ask
Cultural familiarity doesn’t equate with stereotyping, Wheat emphasized. “Proceeding without assumptions opens the opportunity to ask questions for clarification and understanding and to improve patient care,” said Lee.
Sara Glass, PhD, LCSW, agrees. She’s the clinical director of Soul Wellness NYC, New York City, a psychotherapy practice that specializes in treating trauma. Based on her own experiences, she knows that some physicians and other healthcare professionals confuse cultural sensitivity with cultural stereotyping.
Glass, formerly Hasidic and ultra-Orthodox, shared an example from her own life. During the delivery of her second child, she sustained a vaginal tear. At her 6-week postpartum visit, her ob/gyn said, “Just remind me when you’re in your ninth month next time, and I can sew it up right after you deliver.”
Much of this physician’s practice “consisted of Hasidic women who looked just like me, wearing the same garb — head coverings such as wigs and scarves and long skirts. Most women in that community have multiple pregnancies,” Glass told this news organization. “My sister has 10 children, and that’s not unusual. The doctor simply assumed I’d be going on to have more babies without asking if that’s what I wanted.”
Glass says she was also never given information by her physician about the range of available contraceptive options. The rabbis of the Hasidic sect to which Glass belonged allowed women to practice contraception for 6 months following childbirth, or for longer, in the setting of certain medical conditions, but only certain types of birth control were religiously permissible. Other options were not mentioned to her by her physician, and she didn’t know that they existed.
Making no assumptions applies not only to patients from other cultures but also to all patients — including members of “mainstream American culture.”
Candler recalls a young patient with a new baby, who shared “how exhausted she was and how much time, energy, and work it took to care for children,” Candler recounted. “To me, it sounded as though she didn’t want another child, and I was about to offer contraception when it occurred to me to first ask if she wanted to have more children.” Candler was surprised when the patient said that, although she wasn’t actively looking to become pregnant again, she didn’t want to take preventive measures. “I’m so glad I asked, rather than simply assuming.”
Culture Is Mutable
Important questions to ask patients include whether there are aspects of their culture or religion that might affect their care — which can include medications they may feel uncomfortable using — and what family members they want to have involved in clinical discussions and decisions, said Wheat.
Lee described treating a refugee from Afghanistan who was in her sixth month of pregnancy. “I quickly needed to learn about what her expectations were for her care and my presence as a male on her care team,” he recounted. Lee arranged for the patient to receive prenatal care from a different clinician and arranged for follow-up for her husband and children. “Everyone had good results.”
Candler noted that some patients choose their physician specifically because that practitioner is conversant with their culture and respectful of its mores — especially when physicians share the same culture as the patient. But that level of familiarity can make it easy to forget to ask questions about the experience of the individual patient within that culture.
Moreover, Glass suggested, some physicians who treat patients from a particular culture or religious group may be concerned about offending them or antagonizing religious leaders if they discuss medical options that aren’t accepted or practiced in that community or culture, such as vasectomy for male contraception. “But that deprives patients of knowing what choices are available and making truly informed decisions.”
This is especially important because “culture is mutable,” said Candler, and religious or cultural practices can “look one way on paper but be implemented, adopted, or executed in a completely different way by every human being who lives in that culture.” The best cultural competency “comes from continuing to build relationships with our patients. But even in a single visit, a single hospitalization, we should get to know patients as human beings, not just members of a given culture.”
There are cultures in which families want to be the liaison between the patient and the physician and to make decisions on the patient’s behalf. “I always ask patients what role they want their family members to play even if the cultural expectation is that the family will be heavily involved,” Candler said.
Sometimes, this can be awkward, and families might become upset. Candler described an elderly, frail patient who was diagnosed with end-stage cancer. She had always relied heavily on family to care for her. Concerned about overburdening them, she didn’t want them to know her diagnosis. The patient was mentally competent to make that decision.
“Usually, I would have had the family at the bedside so I could be sure everyone was appropriately informed and prepared for what lay ahead, but in this case, I couldn’t do so,” Candler said. “I had to inform her entire care team not to discuss the cancer diagnosis with any family members because this was the patient’s express wish. And when the family asked me if the diagnosis was cancer, I had to respond, ‘I’m so sorry, but your loved one doesn’t want us to discuss details of her diagnosis.’”
Other patients don’t want to know their own diagnosis and specifically ask Candler to inform a family member. “I’ve had patients request that I tell their children. They want their children to make decisions on their behalf.”
The main thing, Candler emphasized, is to “ask the patient, make sure the patient is competent to make that decision, thoroughly document the patient’s decision in the chart, and respect whatever that decision is.”
You Can Revisit the Questions
Having a longitudinal relationship means that the physician can revisit the same questions at different junctures because people’s perspectives sometimes change over time. “Discussing what a patient wants isn’t necessarily a one-time occurrence,” Wheat said. For example, “I’ve had situations where a patient has been a member of Jehovah’s Witnesses and won’t accept blood products — like transfusions — in treatment. I tell these patients that if an emergent situation arises, I would like to have the conversation again.”
Of course, sometimes patients are seen in the emergency department or in other situations where the physician has no prior relationship with them. “I always go into a room, especially with new patients, aiming to build rapport, communicate with a high level of respect, introduce myself, explain my approach, and understand the patient’s wishes,” Lee said. “As scenarios play out, I ask in multiple ways for the patient to confirm those wishes.”
He acknowledges that this can be time-consuming, “but it helps ensure the care that patient receives is complete, thorough, comprehensive, and respectful of the patient’s values and wishes.”
Candler disclosed paid part-time clinical work at CuraCapitol Primary Care Services, volunteer advocacy (reimbursed for travel) for the American College of Physicians, volunteer advocacy (reimbursed for travel) for the American Medical Association while serving on their Task Force to Preserve the Patient-Physician Relationship, and serving as a partner representative (reimbursed for time) for the AHRQ’s Person-Centered Care Planning Partnership, representing the American College of Physicians. Lee, Wheat, and Glass disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Cultural competency is one of the most important values in the practice of medicine. Defined as the “ability to collaborate effectively with individuals from different cultures,” this type of competence “improves healthcare experiences and outcomes.” But within the context of cultural familiarity, it’s equally important to “understand that each person is an individual and may or may not adhere to certain cultural beliefs or practices common in his or her culture,” according to the Agency for Healthcare Research and Quality’s (AHRQ’s) Health Literacy Universal Precautions Toolkit.
Sarah Candler, MD, MPH, an internal medicine physician specializing in primary care for older adults in Washington, DC, said that the medical code of ethics consists of several pillars, with patient autonomy as the “first and most primary of those pillars.” She calls the balance of patient autonomy and cultural respect a “complicated tightrope to walk,” but says that these ethical principles can inform medical decisions and the patient-physician relationship.
Cultural Familiarity
It’s important to be as familiar as possible with the patient’s culture, Santina Wheat, MD, program director, Northwestern McGaw Family Medicine Residency at Delnor Hospital, Geneva, told this news organization. “For example, we serve many Orthodox Jewish patients. We had a meeting with rabbis from the community to present to us what religious laws might affect our patients. Until recently, I was delivering babies, and there was always a 24-hour emergency rabbi on call if an Orthodox patient wanted the input of a rabbi into her decisions.”
Jay W. Lee, MD, MPH, a member of the board of directors of the American Academy of Family Physicians, also sets out to educate himself about the cultural norms of his patients if they come from populations he’s not familiar with. “For example, this comes up when a new refugee population comes to the United States — most recently, there was a population of Afghan refugees,” Lee told this news organization.
Lee spent “a lot of time trying to learn about their cultural norms,” which prepared him to “ask more targeted questions about the patient’s understanding of the tests we were ordering or treatment options we were bringing forward.”
Lee, also the medical director at Integrated Health Partners of Southern California and associate clinical professor of family medicine at the University of California, Irvine, said it might be best if the physician is “language congruent or culturally similar.” Lee is of Asian descent and also speaks Spanish fluently. “I enjoy cultural exchanges with my patients, and I encourage patients to find a physician who’s the best fit.” But being from the same culture isn’t absolutely necessary for building relationships with the patient. “The key is offering the patient autonomy” while understanding the cultural context.
Don’t Assume ... Always Ask
Cultural familiarity doesn’t equate with stereotyping, Wheat emphasized. “Proceeding without assumptions opens the opportunity to ask questions for clarification and understanding and to improve patient care,” said Lee.
Sara Glass, PhD, LCSW, agrees. She’s the clinical director of Soul Wellness NYC, New York City, a psychotherapy practice that specializes in treating trauma. Based on her own experiences, she knows that some physicians and other healthcare professionals confuse cultural sensitivity with cultural stereotyping.
Glass, formerly Hasidic and ultra-Orthodox, shared an example from her own life. During the delivery of her second child, she sustained a vaginal tear. At her 6-week postpartum visit, her ob/gyn said, “Just remind me when you’re in your ninth month next time, and I can sew it up right after you deliver.”
Much of this physician’s practice “consisted of Hasidic women who looked just like me, wearing the same garb — head coverings such as wigs and scarves and long skirts. Most women in that community have multiple pregnancies,” Glass told this news organization. “My sister has 10 children, and that’s not unusual. The doctor simply assumed I’d be going on to have more babies without asking if that’s what I wanted.”
Glass says she was also never given information by her physician about the range of available contraceptive options. The rabbis of the Hasidic sect to which Glass belonged allowed women to practice contraception for 6 months following childbirth, or for longer, in the setting of certain medical conditions, but only certain types of birth control were religiously permissible. Other options were not mentioned to her by her physician, and she didn’t know that they existed.
Making no assumptions applies not only to patients from other cultures but also to all patients — including members of “mainstream American culture.”
Candler recalls a young patient with a new baby, who shared “how exhausted she was and how much time, energy, and work it took to care for children,” Candler recounted. “To me, it sounded as though she didn’t want another child, and I was about to offer contraception when it occurred to me to first ask if she wanted to have more children.” Candler was surprised when the patient said that, although she wasn’t actively looking to become pregnant again, she didn’t want to take preventive measures. “I’m so glad I asked, rather than simply assuming.”
Culture Is Mutable
Important questions to ask patients include whether there are aspects of their culture or religion that might affect their care — which can include medications they may feel uncomfortable using — and what family members they want to have involved in clinical discussions and decisions, said Wheat.
Lee described treating a refugee from Afghanistan who was in her sixth month of pregnancy. “I quickly needed to learn about what her expectations were for her care and my presence as a male on her care team,” he recounted. Lee arranged for the patient to receive prenatal care from a different clinician and arranged for follow-up for her husband and children. “Everyone had good results.”
Candler noted that some patients choose their physician specifically because that practitioner is conversant with their culture and respectful of its mores — especially when physicians share the same culture as the patient. But that level of familiarity can make it easy to forget to ask questions about the experience of the individual patient within that culture.
Moreover, Glass suggested, some physicians who treat patients from a particular culture or religious group may be concerned about offending them or antagonizing religious leaders if they discuss medical options that aren’t accepted or practiced in that community or culture, such as vasectomy for male contraception. “But that deprives patients of knowing what choices are available and making truly informed decisions.”
This is especially important because “culture is mutable,” said Candler, and religious or cultural practices can “look one way on paper but be implemented, adopted, or executed in a completely different way by every human being who lives in that culture.” The best cultural competency “comes from continuing to build relationships with our patients. But even in a single visit, a single hospitalization, we should get to know patients as human beings, not just members of a given culture.”
There are cultures in which families want to be the liaison between the patient and the physician and to make decisions on the patient’s behalf. “I always ask patients what role they want their family members to play even if the cultural expectation is that the family will be heavily involved,” Candler said.
Sometimes, this can be awkward, and families might become upset. Candler described an elderly, frail patient who was diagnosed with end-stage cancer. She had always relied heavily on family to care for her. Concerned about overburdening them, she didn’t want them to know her diagnosis. The patient was mentally competent to make that decision.
“Usually, I would have had the family at the bedside so I could be sure everyone was appropriately informed and prepared for what lay ahead, but in this case, I couldn’t do so,” Candler said. “I had to inform her entire care team not to discuss the cancer diagnosis with any family members because this was the patient’s express wish. And when the family asked me if the diagnosis was cancer, I had to respond, ‘I’m so sorry, but your loved one doesn’t want us to discuss details of her diagnosis.’”
Other patients don’t want to know their own diagnosis and specifically ask Candler to inform a family member. “I’ve had patients request that I tell their children. They want their children to make decisions on their behalf.”
The main thing, Candler emphasized, is to “ask the patient, make sure the patient is competent to make that decision, thoroughly document the patient’s decision in the chart, and respect whatever that decision is.”
You Can Revisit the Questions
Having a longitudinal relationship means that the physician can revisit the same questions at different junctures because people’s perspectives sometimes change over time. “Discussing what a patient wants isn’t necessarily a one-time occurrence,” Wheat said. For example, “I’ve had situations where a patient has been a member of Jehovah’s Witnesses and won’t accept blood products — like transfusions — in treatment. I tell these patients that if an emergent situation arises, I would like to have the conversation again.”
Of course, sometimes patients are seen in the emergency department or in other situations where the physician has no prior relationship with them. “I always go into a room, especially with new patients, aiming to build rapport, communicate with a high level of respect, introduce myself, explain my approach, and understand the patient’s wishes,” Lee said. “As scenarios play out, I ask in multiple ways for the patient to confirm those wishes.”
He acknowledges that this can be time-consuming, “but it helps ensure the care that patient receives is complete, thorough, comprehensive, and respectful of the patient’s values and wishes.”
Candler disclosed paid part-time clinical work at CuraCapitol Primary Care Services, volunteer advocacy (reimbursed for travel) for the American College of Physicians, volunteer advocacy (reimbursed for travel) for the American Medical Association while serving on their Task Force to Preserve the Patient-Physician Relationship, and serving as a partner representative (reimbursed for time) for the AHRQ’s Person-Centered Care Planning Partnership, representing the American College of Physicians. Lee, Wheat, and Glass disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Program Director Perspectives on DEI Initiatives in the Dermatology Residency Selection Process
Program Director Perspectives on DEI Initiatives in the Dermatology Residency Selection Process
The recent Supreme Court ruling that struck down affirmative action1 has caused many initiatives aimed at promoting diversity, equity, and inclusion (DEI) to fall under scrutiny; however, the American Academy of Dermatology (AAD) published a statement of intent in 2022 recognizing and committing to DEI as a priority in the specialty.2 In this study, we used a formal survey to investigate the perceptions of dermatology program directors (PDs) on DEI programming from the AAD and how DEI is integrated into the resident selection process at varying institutions.
Methods
We conducted a cross-sectional study of dermatology PDs across the United States from April 2024 to July 2024. Program directors were contacted via the Association of Professors of Dermatology PD listserve, which includes all 103 PDs who are members of the organization. Personalized survey links were created and sent individually to each PD’s email address. Thirty responses were received. All survey responses were captured anonymously. The survey consisted of 17 questions focusing on dermatology PD demographics and opinions on DEI initiatives in the AAD and in the dermatology resident selection process. Data were collected using Qualtrics survey tools and analyzed using Qualtrics reports.
Results
Demographics—A total of 30 completed surveys were received. Thirty-three percent (10/30) of respondents were from the Midwest, and 23% (7/30) were from the Northeast. The next most represented region was the West, with 20% (6/30) of respondents. The Southeast and Southwest were the least represented regions captured in our survey, accounting for 13% (4/30) and 10% (3/30) of respondents, respectively. After answering this initial demographic question, 1 respondent stopped the survey, bringing our new total to 29 respondents.
Most (66% [19/29]) of the survey respondents had served as PDs for 5 years or less. Sixty-nine percent (20/29) identified as female, while 31% (9/29) identified as male. Seventy-two percent (21/29) identified as White, 17% (5/29) identified as Asian, 3% (1/29) identified as Black/African American, 3% (1/29) identified as Hispanic or Latinx, and 3% (1/29) identified as mixed race.
Opinions on DEI Initiatives—When asked about their satisfaction level with the current amount of DEI efforts within the AAD, 17% (5/29) of respondents said they were very satisfied, 59% (17/29) said they were satisfied, 17% (5/29) said they were neutral, and 7% (2/29) said they were dissatisfied. Given that none of the questions were mandatory to answer before proceeding with the survey, there were variable response rates to each of the remaining questions, which may have caused respondents to answer only questions they felt strongly about.
Twenty respondents answered when prompted to further classify their level of satisfaction: 70% (14/20) said there should be more DEI efforts through the AAD providing financial support, and 50% (10/20) wanted more nonfinancial support. When given the opportunity to specify which DEI initiatives should be enhanced, the majority (67% [14/21]) of PDs chose the AAD’s health disparities curriculum, followed by the Diversity Mentorship Program (52% [11/21]), AAD Diversity Toolkit (43% [9/21]), and the Skin of Color Curriculum (43% [9/21]). Thiry-three percent (7/30) of PDs wanted enhancement of Medicine Without Barriers: Overcoming Unintended Bias in Practice (an AAD educational resource), and 19% (4/21) of respondents did not think any of the AAD’s DEI initiatives needed to be enhanced. There were 14 responses to a question about choosing which DEI initiatives to reduce with singular votes (7% [1/14] each) to reduce Medicine Without Barriers: Overcoming Unintended Bias in Practice and the Skin of Color Curriculum.
Our survey also invited PDs to introduce ideas for new DEI initiatives or programs. The following were suggestions offered by respondents: education for senior members of the AAD on the importance of DEI in dermatology, professional development resources directed toward academic faculty members to prepare them for interacting with and teaching residents from different backgrounds, and more advertisements and support for the AAD’s Diversity Champion Workshop.
DEI in Resident Selection—When asked about the role that DEI plays in how programs develop their match lists for residency, 13% (3/23) of PDs responded that it plays a very large role, 52% (12/23) stated that it plays a large role, 26% (6/23) responded that it plays somewhat of a role, 4% (1/23) stated that it plays a small role, and 4% (1/23) stated that it plays no role. Twenty-four percent (4/17) of respondents were PDs in states that have legislation limiting or defunding DEI initiatives at institutions of higher education. Another 12% (2/17) were from states where such legislation was pending a vote, while 59% (10/17) of respondents indicated that their state had not introduced such legislation. Four percent (1/17) indicated that they were from a state that had introduced legislation to limit or defund DEI initiatives that failed to pass. Only 17 respondents answered this question, which may be due to a lack of awareness among respondents of state-specific legislation on limiting or defunding DEI initiatives.
Resident Selection Factors—Ninety-six percent (22/23) of PDs stated that their residency program uses a holistic review that takes into account factors such as experiences (eg, volunteer work, research endeavors), personal attributes, and metrics in a balanced manner. No PDs offered United States Medical Licensing Examination Step score cutoffs or medical school clerkship cutoff grades. When asked to rank the importance placed on individual factors in the residency application, the following were ranked from most to least important in the process: performance on clerkships/rotations, performance on interviews, letters of recommendation, clerkship grades, United States Medical Licensing Examination Step scores, research content/ quality, race/ethnicity, history of teaching and mentorship, volunteering, and research amount. When asked to indicate the most pertinent factors used to incorporate DEI in resident selection, the most popular factor was lived experience/life, which was chosen by 90% (18/20) of PDs followed by 75% (15/20) of respondents incorporating underrepresented in medicine (URM) status (including Black, Latinx, and Native American applicants) and 70% (4/20) incorporating socioeconomic status. Sexual orientation and geographic ties of the applicant to the region of the residency program was incorporated by 45% (9/20) of respondents, and other characteristics of race and sex each were incorporated by 30% (6/20) of respondents. Religion was the least incorporated, with 10% (2/20) of PDs selecting this classification. In considering URM status when choosing dermatology residents, 100% (11/11) of respondents indicated that their institution promotes diversity as a part of the recruitment process. Eighty-two percent (9/11) of respondents try to recruit URM applicants to reflect their patient population, 82% (9/11) try as part of a belief that a diverse group benefits everyone in their program, and 45% (5/11) try in order to address societal inequities and as a broader mission to diversify the health care workforce. Seventy-three percent (8/11) indicated that they pay attention to URM status throughout the application process.
Comment
Diversity in the US population is steadily increasing. Within the past decade, the diversity index (the probability that 2 people chosen at random will be from different racial and ethnic groups) has grown from 54.9% in 2010 to 61.1% in 2020.3 There was a 24.9% increase in population groups other than non-Hispanic Whites from 2010 to 2020, an increase in diversity that was present in every region of the United States.4 The field of dermatology already does not reflect the racial distribution of the nation,4 with Black individuals accounting for 13.7% of the nation’s population but only 3% of dermatologists; similarly, Hispanic individuals account for 19.5% of the population but only comprise 4.2% of dermatologists.5,6 There is overwhelming evidence that patients prefer to be diagnosed and treated by physicians who reflect their own demographics.7 Furthermore, physicians who prescribe treatment plans that reflect and respect socioeconomic and religious beliefs of the populations they serve enable patients to meet treatment expectations and experience better outcomes.8 Direct action is required to ensure that the specialty more accurately represents the evolving demographics of the country. This can be accomplished in myriad ways, including but not limited to cultural humility training9 for current dermatologists and trainees and recruitment of a more diverse workforce. These measures can ultimately improve treatment approaches and outcomes for dermatologic conditions across various groups.10
There are efforts by various dermatologic organizations, including the AAD, Society for Pediatric Dermatology, Pediatric Dermatology Research Alliance, Skin of Color Society, Women’s Dermatologic Society, and American Society for Dermatologic Surgery, that are focused on promoting DEI through research, education, and mentorship of potential future dermatologists.11 However, the perceptions, opinions, and selection process instituted by PDs are most consequential in determining the diversity of the specialty, as PDs are at the forefront of establishing the next generation of dermatologists. Through this study, we have found that most PDs recognize the importance of diversity in residency education and recruitment without it being the only deciding factor.
The main limitation of this study was the small sample size, which may not adequately represent all dermatology residency programs accredited by the Accreditation Council for Graduate Medical Education as a result of selection bias toward respondents who were more likely to participate in survey-based research on topics of DEI.
Conclusion
This study revealed that, among dermatology residency PDs, there is interest in modifying the resources and initiatives surrounding DEI in the field. It also revealed that DEI remains a consideration in the resident selection process despite the recent Supreme Court ruling. In conclusion, there is an eagerness among dermatology PDs to incorporate DEI into resident selection even though gaps in knowledge and awareness remain.
- Supreme Court of the United States. Students for Fair Admissions, Inc v President and Fellows of Harvard College (No. 20–1199). Argued October 31, 2022. Decided June 29, 2023. https://www.supremecourt.gov/opinions/22pdf/20-1199_hgdj.pdf
- American Academy of Dermatology. AAD’s DEI Statement of Intent. Published March 28, 2022. Accessed November 18, 2024. https://www.aad.org/member/career/diversity/diversity-statement-of-intent
- Jensen E, Jones N, Rabe M, et al. The chance that two people chosen at random are of different race or ethnicity groups has increased since 2010. United States Census Bureau. August 12, 2021. Accessed November 5, 2024. https://www.census.gov/library/stories/2021/08/2020-united-states-population-more-racially-ethnically-diverse-than-2010.html
- Johnson K. New Census reflects growing U.S. population diversity, with children in the forefront. University of New Hampshire Carsey School of Public Policy. October 6, 2021. Accessed November 5, 2024. https://carsey.unh.edu/publication/new-census-reflects-growing-us-population-diversity-children-forefront
- Pandya AG, Alexis AF, Berger TG, et al. Increasing racial and ethnic diversity in dermatology: a call to action. J Am Acad Dermatol. 2016;74; 584-587. doi:10.1016/j.jaad.2015.10.044
- United States Census Bureau. QuickFacts: United States. Population estimates, July 1, 2023 (V2023). Accessed November 5, 2024. https://www.census.gov/quickfacts/fact/table/US/PST045222
- Saha S, Beach MC. Impact of physician race on patient decision-making and ratings of physicians: a randomized experiment using video vignettes. J Gen Intern Med. 2020;35:1084-1091. doi:10.1007/s11606-020-05646-z
- Nair L, Adetayo OA. Cultural competence and ethnic diversity in healthcare. Plast Reconstr Surg Glob Open. 2019;7:E2219. doi:10.1097/GOX.0000000000002219
- Yeager KA, Bauer-Wu S. Cultural humility: essential foundation for clinical researchers. Appl Nurs Res. 2013;26:251-256. doi:10.1016/j.apnr.2013.06.008
- Narla S, Heath CR, Alexis A, et al. Racial disparities in dermatology. Arch Dermatol Res. 2023;315:1215-1223. doi:10.1007/s00403-022- 02507-z
- Desai SR, Khanna R, Glass D, et al. Embracing diversity in dermatology: creation of a culture of equity and inclusion in dermatology. Int J Womens Dermatol. 2021;7:378-382. doi:10.1016/j.ijwd.2021.08.002
The recent Supreme Court ruling that struck down affirmative action1 has caused many initiatives aimed at promoting diversity, equity, and inclusion (DEI) to fall under scrutiny; however, the American Academy of Dermatology (AAD) published a statement of intent in 2022 recognizing and committing to DEI as a priority in the specialty.2 In this study, we used a formal survey to investigate the perceptions of dermatology program directors (PDs) on DEI programming from the AAD and how DEI is integrated into the resident selection process at varying institutions.
Methods
We conducted a cross-sectional study of dermatology PDs across the United States from April 2024 to July 2024. Program directors were contacted via the Association of Professors of Dermatology PD listserve, which includes all 103 PDs who are members of the organization. Personalized survey links were created and sent individually to each PD’s email address. Thirty responses were received. All survey responses were captured anonymously. The survey consisted of 17 questions focusing on dermatology PD demographics and opinions on DEI initiatives in the AAD and in the dermatology resident selection process. Data were collected using Qualtrics survey tools and analyzed using Qualtrics reports.
Results
Demographics—A total of 30 completed surveys were received. Thirty-three percent (10/30) of respondents were from the Midwest, and 23% (7/30) were from the Northeast. The next most represented region was the West, with 20% (6/30) of respondents. The Southeast and Southwest were the least represented regions captured in our survey, accounting for 13% (4/30) and 10% (3/30) of respondents, respectively. After answering this initial demographic question, 1 respondent stopped the survey, bringing our new total to 29 respondents.
Most (66% [19/29]) of the survey respondents had served as PDs for 5 years or less. Sixty-nine percent (20/29) identified as female, while 31% (9/29) identified as male. Seventy-two percent (21/29) identified as White, 17% (5/29) identified as Asian, 3% (1/29) identified as Black/African American, 3% (1/29) identified as Hispanic or Latinx, and 3% (1/29) identified as mixed race.
Opinions on DEI Initiatives—When asked about their satisfaction level with the current amount of DEI efforts within the AAD, 17% (5/29) of respondents said they were very satisfied, 59% (17/29) said they were satisfied, 17% (5/29) said they were neutral, and 7% (2/29) said they were dissatisfied. Given that none of the questions were mandatory to answer before proceeding with the survey, there were variable response rates to each of the remaining questions, which may have caused respondents to answer only questions they felt strongly about.
Twenty respondents answered when prompted to further classify their level of satisfaction: 70% (14/20) said there should be more DEI efforts through the AAD providing financial support, and 50% (10/20) wanted more nonfinancial support. When given the opportunity to specify which DEI initiatives should be enhanced, the majority (67% [14/21]) of PDs chose the AAD’s health disparities curriculum, followed by the Diversity Mentorship Program (52% [11/21]), AAD Diversity Toolkit (43% [9/21]), and the Skin of Color Curriculum (43% [9/21]). Thiry-three percent (7/30) of PDs wanted enhancement of Medicine Without Barriers: Overcoming Unintended Bias in Practice (an AAD educational resource), and 19% (4/21) of respondents did not think any of the AAD’s DEI initiatives needed to be enhanced. There were 14 responses to a question about choosing which DEI initiatives to reduce with singular votes (7% [1/14] each) to reduce Medicine Without Barriers: Overcoming Unintended Bias in Practice and the Skin of Color Curriculum.
Our survey also invited PDs to introduce ideas for new DEI initiatives or programs. The following were suggestions offered by respondents: education for senior members of the AAD on the importance of DEI in dermatology, professional development resources directed toward academic faculty members to prepare them for interacting with and teaching residents from different backgrounds, and more advertisements and support for the AAD’s Diversity Champion Workshop.
DEI in Resident Selection—When asked about the role that DEI plays in how programs develop their match lists for residency, 13% (3/23) of PDs responded that it plays a very large role, 52% (12/23) stated that it plays a large role, 26% (6/23) responded that it plays somewhat of a role, 4% (1/23) stated that it plays a small role, and 4% (1/23) stated that it plays no role. Twenty-four percent (4/17) of respondents were PDs in states that have legislation limiting or defunding DEI initiatives at institutions of higher education. Another 12% (2/17) were from states where such legislation was pending a vote, while 59% (10/17) of respondents indicated that their state had not introduced such legislation. Four percent (1/17) indicated that they were from a state that had introduced legislation to limit or defund DEI initiatives that failed to pass. Only 17 respondents answered this question, which may be due to a lack of awareness among respondents of state-specific legislation on limiting or defunding DEI initiatives.
Resident Selection Factors—Ninety-six percent (22/23) of PDs stated that their residency program uses a holistic review that takes into account factors such as experiences (eg, volunteer work, research endeavors), personal attributes, and metrics in a balanced manner. No PDs offered United States Medical Licensing Examination Step score cutoffs or medical school clerkship cutoff grades. When asked to rank the importance placed on individual factors in the residency application, the following were ranked from most to least important in the process: performance on clerkships/rotations, performance on interviews, letters of recommendation, clerkship grades, United States Medical Licensing Examination Step scores, research content/ quality, race/ethnicity, history of teaching and mentorship, volunteering, and research amount. When asked to indicate the most pertinent factors used to incorporate DEI in resident selection, the most popular factor was lived experience/life, which was chosen by 90% (18/20) of PDs followed by 75% (15/20) of respondents incorporating underrepresented in medicine (URM) status (including Black, Latinx, and Native American applicants) and 70% (4/20) incorporating socioeconomic status. Sexual orientation and geographic ties of the applicant to the region of the residency program was incorporated by 45% (9/20) of respondents, and other characteristics of race and sex each were incorporated by 30% (6/20) of respondents. Religion was the least incorporated, with 10% (2/20) of PDs selecting this classification. In considering URM status when choosing dermatology residents, 100% (11/11) of respondents indicated that their institution promotes diversity as a part of the recruitment process. Eighty-two percent (9/11) of respondents try to recruit URM applicants to reflect their patient population, 82% (9/11) try as part of a belief that a diverse group benefits everyone in their program, and 45% (5/11) try in order to address societal inequities and as a broader mission to diversify the health care workforce. Seventy-three percent (8/11) indicated that they pay attention to URM status throughout the application process.
Comment
Diversity in the US population is steadily increasing. Within the past decade, the diversity index (the probability that 2 people chosen at random will be from different racial and ethnic groups) has grown from 54.9% in 2010 to 61.1% in 2020.3 There was a 24.9% increase in population groups other than non-Hispanic Whites from 2010 to 2020, an increase in diversity that was present in every region of the United States.4 The field of dermatology already does not reflect the racial distribution of the nation,4 with Black individuals accounting for 13.7% of the nation’s population but only 3% of dermatologists; similarly, Hispanic individuals account for 19.5% of the population but only comprise 4.2% of dermatologists.5,6 There is overwhelming evidence that patients prefer to be diagnosed and treated by physicians who reflect their own demographics.7 Furthermore, physicians who prescribe treatment plans that reflect and respect socioeconomic and religious beliefs of the populations they serve enable patients to meet treatment expectations and experience better outcomes.8 Direct action is required to ensure that the specialty more accurately represents the evolving demographics of the country. This can be accomplished in myriad ways, including but not limited to cultural humility training9 for current dermatologists and trainees and recruitment of a more diverse workforce. These measures can ultimately improve treatment approaches and outcomes for dermatologic conditions across various groups.10
There are efforts by various dermatologic organizations, including the AAD, Society for Pediatric Dermatology, Pediatric Dermatology Research Alliance, Skin of Color Society, Women’s Dermatologic Society, and American Society for Dermatologic Surgery, that are focused on promoting DEI through research, education, and mentorship of potential future dermatologists.11 However, the perceptions, opinions, and selection process instituted by PDs are most consequential in determining the diversity of the specialty, as PDs are at the forefront of establishing the next generation of dermatologists. Through this study, we have found that most PDs recognize the importance of diversity in residency education and recruitment without it being the only deciding factor.
The main limitation of this study was the small sample size, which may not adequately represent all dermatology residency programs accredited by the Accreditation Council for Graduate Medical Education as a result of selection bias toward respondents who were more likely to participate in survey-based research on topics of DEI.
Conclusion
This study revealed that, among dermatology residency PDs, there is interest in modifying the resources and initiatives surrounding DEI in the field. It also revealed that DEI remains a consideration in the resident selection process despite the recent Supreme Court ruling. In conclusion, there is an eagerness among dermatology PDs to incorporate DEI into resident selection even though gaps in knowledge and awareness remain.
The recent Supreme Court ruling that struck down affirmative action1 has caused many initiatives aimed at promoting diversity, equity, and inclusion (DEI) to fall under scrutiny; however, the American Academy of Dermatology (AAD) published a statement of intent in 2022 recognizing and committing to DEI as a priority in the specialty.2 In this study, we used a formal survey to investigate the perceptions of dermatology program directors (PDs) on DEI programming from the AAD and how DEI is integrated into the resident selection process at varying institutions.
Methods
We conducted a cross-sectional study of dermatology PDs across the United States from April 2024 to July 2024. Program directors were contacted via the Association of Professors of Dermatology PD listserve, which includes all 103 PDs who are members of the organization. Personalized survey links were created and sent individually to each PD’s email address. Thirty responses were received. All survey responses were captured anonymously. The survey consisted of 17 questions focusing on dermatology PD demographics and opinions on DEI initiatives in the AAD and in the dermatology resident selection process. Data were collected using Qualtrics survey tools and analyzed using Qualtrics reports.
Results
Demographics—A total of 30 completed surveys were received. Thirty-three percent (10/30) of respondents were from the Midwest, and 23% (7/30) were from the Northeast. The next most represented region was the West, with 20% (6/30) of respondents. The Southeast and Southwest were the least represented regions captured in our survey, accounting for 13% (4/30) and 10% (3/30) of respondents, respectively. After answering this initial demographic question, 1 respondent stopped the survey, bringing our new total to 29 respondents.
Most (66% [19/29]) of the survey respondents had served as PDs for 5 years or less. Sixty-nine percent (20/29) identified as female, while 31% (9/29) identified as male. Seventy-two percent (21/29) identified as White, 17% (5/29) identified as Asian, 3% (1/29) identified as Black/African American, 3% (1/29) identified as Hispanic or Latinx, and 3% (1/29) identified as mixed race.
Opinions on DEI Initiatives—When asked about their satisfaction level with the current amount of DEI efforts within the AAD, 17% (5/29) of respondents said they were very satisfied, 59% (17/29) said they were satisfied, 17% (5/29) said they were neutral, and 7% (2/29) said they were dissatisfied. Given that none of the questions were mandatory to answer before proceeding with the survey, there were variable response rates to each of the remaining questions, which may have caused respondents to answer only questions they felt strongly about.
Twenty respondents answered when prompted to further classify their level of satisfaction: 70% (14/20) said there should be more DEI efforts through the AAD providing financial support, and 50% (10/20) wanted more nonfinancial support. When given the opportunity to specify which DEI initiatives should be enhanced, the majority (67% [14/21]) of PDs chose the AAD’s health disparities curriculum, followed by the Diversity Mentorship Program (52% [11/21]), AAD Diversity Toolkit (43% [9/21]), and the Skin of Color Curriculum (43% [9/21]). Thiry-three percent (7/30) of PDs wanted enhancement of Medicine Without Barriers: Overcoming Unintended Bias in Practice (an AAD educational resource), and 19% (4/21) of respondents did not think any of the AAD’s DEI initiatives needed to be enhanced. There were 14 responses to a question about choosing which DEI initiatives to reduce with singular votes (7% [1/14] each) to reduce Medicine Without Barriers: Overcoming Unintended Bias in Practice and the Skin of Color Curriculum.
Our survey also invited PDs to introduce ideas for new DEI initiatives or programs. The following were suggestions offered by respondents: education for senior members of the AAD on the importance of DEI in dermatology, professional development resources directed toward academic faculty members to prepare them for interacting with and teaching residents from different backgrounds, and more advertisements and support for the AAD’s Diversity Champion Workshop.
DEI in Resident Selection—When asked about the role that DEI plays in how programs develop their match lists for residency, 13% (3/23) of PDs responded that it plays a very large role, 52% (12/23) stated that it plays a large role, 26% (6/23) responded that it plays somewhat of a role, 4% (1/23) stated that it plays a small role, and 4% (1/23) stated that it plays no role. Twenty-four percent (4/17) of respondents were PDs in states that have legislation limiting or defunding DEI initiatives at institutions of higher education. Another 12% (2/17) were from states where such legislation was pending a vote, while 59% (10/17) of respondents indicated that their state had not introduced such legislation. Four percent (1/17) indicated that they were from a state that had introduced legislation to limit or defund DEI initiatives that failed to pass. Only 17 respondents answered this question, which may be due to a lack of awareness among respondents of state-specific legislation on limiting or defunding DEI initiatives.
Resident Selection Factors—Ninety-six percent (22/23) of PDs stated that their residency program uses a holistic review that takes into account factors such as experiences (eg, volunteer work, research endeavors), personal attributes, and metrics in a balanced manner. No PDs offered United States Medical Licensing Examination Step score cutoffs or medical school clerkship cutoff grades. When asked to rank the importance placed on individual factors in the residency application, the following were ranked from most to least important in the process: performance on clerkships/rotations, performance on interviews, letters of recommendation, clerkship grades, United States Medical Licensing Examination Step scores, research content/ quality, race/ethnicity, history of teaching and mentorship, volunteering, and research amount. When asked to indicate the most pertinent factors used to incorporate DEI in resident selection, the most popular factor was lived experience/life, which was chosen by 90% (18/20) of PDs followed by 75% (15/20) of respondents incorporating underrepresented in medicine (URM) status (including Black, Latinx, and Native American applicants) and 70% (4/20) incorporating socioeconomic status. Sexual orientation and geographic ties of the applicant to the region of the residency program was incorporated by 45% (9/20) of respondents, and other characteristics of race and sex each were incorporated by 30% (6/20) of respondents. Religion was the least incorporated, with 10% (2/20) of PDs selecting this classification. In considering URM status when choosing dermatology residents, 100% (11/11) of respondents indicated that their institution promotes diversity as a part of the recruitment process. Eighty-two percent (9/11) of respondents try to recruit URM applicants to reflect their patient population, 82% (9/11) try as part of a belief that a diverse group benefits everyone in their program, and 45% (5/11) try in order to address societal inequities and as a broader mission to diversify the health care workforce. Seventy-three percent (8/11) indicated that they pay attention to URM status throughout the application process.
Comment
Diversity in the US population is steadily increasing. Within the past decade, the diversity index (the probability that 2 people chosen at random will be from different racial and ethnic groups) has grown from 54.9% in 2010 to 61.1% in 2020.3 There was a 24.9% increase in population groups other than non-Hispanic Whites from 2010 to 2020, an increase in diversity that was present in every region of the United States.4 The field of dermatology already does not reflect the racial distribution of the nation,4 with Black individuals accounting for 13.7% of the nation’s population but only 3% of dermatologists; similarly, Hispanic individuals account for 19.5% of the population but only comprise 4.2% of dermatologists.5,6 There is overwhelming evidence that patients prefer to be diagnosed and treated by physicians who reflect their own demographics.7 Furthermore, physicians who prescribe treatment plans that reflect and respect socioeconomic and religious beliefs of the populations they serve enable patients to meet treatment expectations and experience better outcomes.8 Direct action is required to ensure that the specialty more accurately represents the evolving demographics of the country. This can be accomplished in myriad ways, including but not limited to cultural humility training9 for current dermatologists and trainees and recruitment of a more diverse workforce. These measures can ultimately improve treatment approaches and outcomes for dermatologic conditions across various groups.10
There are efforts by various dermatologic organizations, including the AAD, Society for Pediatric Dermatology, Pediatric Dermatology Research Alliance, Skin of Color Society, Women’s Dermatologic Society, and American Society for Dermatologic Surgery, that are focused on promoting DEI through research, education, and mentorship of potential future dermatologists.11 However, the perceptions, opinions, and selection process instituted by PDs are most consequential in determining the diversity of the specialty, as PDs are at the forefront of establishing the next generation of dermatologists. Through this study, we have found that most PDs recognize the importance of diversity in residency education and recruitment without it being the only deciding factor.
The main limitation of this study was the small sample size, which may not adequately represent all dermatology residency programs accredited by the Accreditation Council for Graduate Medical Education as a result of selection bias toward respondents who were more likely to participate in survey-based research on topics of DEI.
Conclusion
This study revealed that, among dermatology residency PDs, there is interest in modifying the resources and initiatives surrounding DEI in the field. It also revealed that DEI remains a consideration in the resident selection process despite the recent Supreme Court ruling. In conclusion, there is an eagerness among dermatology PDs to incorporate DEI into resident selection even though gaps in knowledge and awareness remain.
- Supreme Court of the United States. Students for Fair Admissions, Inc v President and Fellows of Harvard College (No. 20–1199). Argued October 31, 2022. Decided June 29, 2023. https://www.supremecourt.gov/opinions/22pdf/20-1199_hgdj.pdf
- American Academy of Dermatology. AAD’s DEI Statement of Intent. Published March 28, 2022. Accessed November 18, 2024. https://www.aad.org/member/career/diversity/diversity-statement-of-intent
- Jensen E, Jones N, Rabe M, et al. The chance that two people chosen at random are of different race or ethnicity groups has increased since 2010. United States Census Bureau. August 12, 2021. Accessed November 5, 2024. https://www.census.gov/library/stories/2021/08/2020-united-states-population-more-racially-ethnically-diverse-than-2010.html
- Johnson K. New Census reflects growing U.S. population diversity, with children in the forefront. University of New Hampshire Carsey School of Public Policy. October 6, 2021. Accessed November 5, 2024. https://carsey.unh.edu/publication/new-census-reflects-growing-us-population-diversity-children-forefront
- Pandya AG, Alexis AF, Berger TG, et al. Increasing racial and ethnic diversity in dermatology: a call to action. J Am Acad Dermatol. 2016;74; 584-587. doi:10.1016/j.jaad.2015.10.044
- United States Census Bureau. QuickFacts: United States. Population estimates, July 1, 2023 (V2023). Accessed November 5, 2024. https://www.census.gov/quickfacts/fact/table/US/PST045222
- Saha S, Beach MC. Impact of physician race on patient decision-making and ratings of physicians: a randomized experiment using video vignettes. J Gen Intern Med. 2020;35:1084-1091. doi:10.1007/s11606-020-05646-z
- Nair L, Adetayo OA. Cultural competence and ethnic diversity in healthcare. Plast Reconstr Surg Glob Open. 2019;7:E2219. doi:10.1097/GOX.0000000000002219
- Yeager KA, Bauer-Wu S. Cultural humility: essential foundation for clinical researchers. Appl Nurs Res. 2013;26:251-256. doi:10.1016/j.apnr.2013.06.008
- Narla S, Heath CR, Alexis A, et al. Racial disparities in dermatology. Arch Dermatol Res. 2023;315:1215-1223. doi:10.1007/s00403-022- 02507-z
- Desai SR, Khanna R, Glass D, et al. Embracing diversity in dermatology: creation of a culture of equity and inclusion in dermatology. Int J Womens Dermatol. 2021;7:378-382. doi:10.1016/j.ijwd.2021.08.002
- Supreme Court of the United States. Students for Fair Admissions, Inc v President and Fellows of Harvard College (No. 20–1199). Argued October 31, 2022. Decided June 29, 2023. https://www.supremecourt.gov/opinions/22pdf/20-1199_hgdj.pdf
- American Academy of Dermatology. AAD’s DEI Statement of Intent. Published March 28, 2022. Accessed November 18, 2024. https://www.aad.org/member/career/diversity/diversity-statement-of-intent
- Jensen E, Jones N, Rabe M, et al. The chance that two people chosen at random are of different race or ethnicity groups has increased since 2010. United States Census Bureau. August 12, 2021. Accessed November 5, 2024. https://www.census.gov/library/stories/2021/08/2020-united-states-population-more-racially-ethnically-diverse-than-2010.html
- Johnson K. New Census reflects growing U.S. population diversity, with children in the forefront. University of New Hampshire Carsey School of Public Policy. October 6, 2021. Accessed November 5, 2024. https://carsey.unh.edu/publication/new-census-reflects-growing-us-population-diversity-children-forefront
- Pandya AG, Alexis AF, Berger TG, et al. Increasing racial and ethnic diversity in dermatology: a call to action. J Am Acad Dermatol. 2016;74; 584-587. doi:10.1016/j.jaad.2015.10.044
- United States Census Bureau. QuickFacts: United States. Population estimates, July 1, 2023 (V2023). Accessed November 5, 2024. https://www.census.gov/quickfacts/fact/table/US/PST045222
- Saha S, Beach MC. Impact of physician race on patient decision-making and ratings of physicians: a randomized experiment using video vignettes. J Gen Intern Med. 2020;35:1084-1091. doi:10.1007/s11606-020-05646-z
- Nair L, Adetayo OA. Cultural competence and ethnic diversity in healthcare. Plast Reconstr Surg Glob Open. 2019;7:E2219. doi:10.1097/GOX.0000000000002219
- Yeager KA, Bauer-Wu S. Cultural humility: essential foundation for clinical researchers. Appl Nurs Res. 2013;26:251-256. doi:10.1016/j.apnr.2013.06.008
- Narla S, Heath CR, Alexis A, et al. Racial disparities in dermatology. Arch Dermatol Res. 2023;315:1215-1223. doi:10.1007/s00403-022- 02507-z
- Desai SR, Khanna R, Glass D, et al. Embracing diversity in dermatology: creation of a culture of equity and inclusion in dermatology. Int J Womens Dermatol. 2021;7:378-382. doi:10.1016/j.ijwd.2021.08.002
Program Director Perspectives on DEI Initiatives in the Dermatology Residency Selection Process
Program Director Perspectives on DEI Initiatives in the Dermatology Residency Selection Process
PRACTICE POINTS
- A majority of dermatology program directors (PDs) express support for increased diversity, equity, and inclusion (DEI) funding through the American Academy of Dermatology, including initiatives centered on education and mentorship.
- Dermatology PDs are invested in recruiting underrepresented in medicine applicants to create residency classes that are representative of their patient populations.
Watch That Attitude: Is There Ageism in Healthcare?
People are living longer in Europe. Life expectancy increased on the continent by around 12 years between 1960 and 2022. And despite slower progress during the COVID-19 pandemic, the trend appears to be continuing.
Not only are Europeans living longer, their fertility rates are declining. This means that the number of people aged 75-84 years is projected to grow in Europe a full 56.1% by 2050, while the population younger than 55 years is expected to fall by 13.5%.
This means that attitudes toward age need to change, and fast — even among healthcare professionals.
Healthcare Is Not Exempt From Ageist Attitudes
A systematic review published in the journal PLOS ONE in 2020 found that age was a determinant factor in dictating who received certain medical procedures or treatments. For example, a study of 9105 hospitalized patients found that healthcare providers were significantly more likely to withhold life-sustaining treatments from older patients. Another study found evidence that older people are excluded from clinical trials, even when the trials are for diseases that appear later in life, like Parkinson’s.
“In healthcare, there are different levels of ageism,” explained Hannah Swift, PhD, reader in social and organizational psychology at the University of Kent in the United Kingdom.
Ageism is embedded in the laws, rules, and practices of institutions, she explained. This became especially obvious during the pandemic, when health professionals had to decide who to treat, possibly using age as a proxy for making some of these decisions, she said.
“When you categorize people, you might be using stereotypes, assumptions, and expectations about age and that age group to make those decisions, and that’s where errors can occur.”
She added that ageist attitudes also become apparent at the interpersonal level by using patronizing language or offering unnecessary help to older people based on assumptions about their cognitive and physical abilities.
“Older age is often wrongly associated with declining levels of health and activity,” said Ittay Mannheim, PhD, guest postdoctoral researcher on aging and ageism at the Open University of the Netherlands. “However, older adults are a very diverse group, varying widely in many aspects, including health conditions. This stereotype can influence how healthcare professionals interact with them, assuming frailty or memory issues simply based on age. It’s important to recognize that being older doesn’t necessarily mean being ill.”
Mannheim’s research found that healthcare professionals often stand in the way of older people using technology-based treatments due to negative attitudes towards age. “So, actually, a barrier to using these technologies could be that healthcare professionals don’t think that someone can use it or won’t even offer it because someone looks old or is old,” he said.
The Impacts
Discrimination impacts the physical, mental, and social well-being of its victims. This includes attitudes towards age.
The PLOS ONE review of research on the global reach of ageism found that experienced or self-determined ageism was associated with significantly worse health outcomes across all countries examined. The same research team calculated that an estimated 6.3 million cases of depression worldwide are linked to ageism.
Other research has found that exposure to negative age stereotyping impacts willingness to adopt a healthy lifestyle in addition to increasing the risk for cardiovascular events.
What Can Be Done?
“Healthcare professionals frequently interact with older adults at their most vulnerable, which can reinforce negative stereotypes of older people being vulnerable or ill,” said Swift. “However, not all older adults fit these stereotypes. Many can live well and independently. Perhaps healthcare education should include reminders of the diverse experiences of older individuals rather than solely focusing on the moments when they require help.”
Research indicates that although progress has been made in geriatric training and the care of older individuals by healthcare education institutions, improved education and training are still needed at all levels of geriatric healthcare, including hospital administrators, physicians, nurses, personal caregivers, and associated health professions.
“Generally speaking, what healthcare professionals learn about aging tends to focus more on the biological aspects,” said Mannheim. “However, they may not fully understand what it means to be old or how to interact with older individuals, especially regarding technology. It is important to raise awareness about ageism because, in my experience working with healthcare professionals, even a single workshop on ageism can have a profound impact. Participants often respond with surprise, saying something like, ‘Wow, I never thought about this before.’”
Mannheim said that training healthcare providers to understand the aging process better could help to reduce any biases they might have and better prepare them to respond more adequately to the needs of older patients.
“We cannot devalue the lives of older people simply because they are older. It is crucial for all of us, especially governments, to acknowledge our responsibility to protect and promote human rights for individuals of all ages. If we fail to do this, the strategies we’ve witnessed during this pandemic will be repeated in the future,” said Nena Georgantzi, PhD, Barcelona-based human rights manager at AGE Platform Europe, an EU network of organizations of and for older people.
A version of this article appeared on Medscape.com.
People are living longer in Europe. Life expectancy increased on the continent by around 12 years between 1960 and 2022. And despite slower progress during the COVID-19 pandemic, the trend appears to be continuing.
Not only are Europeans living longer, their fertility rates are declining. This means that the number of people aged 75-84 years is projected to grow in Europe a full 56.1% by 2050, while the population younger than 55 years is expected to fall by 13.5%.
This means that attitudes toward age need to change, and fast — even among healthcare professionals.
Healthcare Is Not Exempt From Ageist Attitudes
A systematic review published in the journal PLOS ONE in 2020 found that age was a determinant factor in dictating who received certain medical procedures or treatments. For example, a study of 9105 hospitalized patients found that healthcare providers were significantly more likely to withhold life-sustaining treatments from older patients. Another study found evidence that older people are excluded from clinical trials, even when the trials are for diseases that appear later in life, like Parkinson’s.
“In healthcare, there are different levels of ageism,” explained Hannah Swift, PhD, reader in social and organizational psychology at the University of Kent in the United Kingdom.
Ageism is embedded in the laws, rules, and practices of institutions, she explained. This became especially obvious during the pandemic, when health professionals had to decide who to treat, possibly using age as a proxy for making some of these decisions, she said.
“When you categorize people, you might be using stereotypes, assumptions, and expectations about age and that age group to make those decisions, and that’s where errors can occur.”
She added that ageist attitudes also become apparent at the interpersonal level by using patronizing language or offering unnecessary help to older people based on assumptions about their cognitive and physical abilities.
“Older age is often wrongly associated with declining levels of health and activity,” said Ittay Mannheim, PhD, guest postdoctoral researcher on aging and ageism at the Open University of the Netherlands. “However, older adults are a very diverse group, varying widely in many aspects, including health conditions. This stereotype can influence how healthcare professionals interact with them, assuming frailty or memory issues simply based on age. It’s important to recognize that being older doesn’t necessarily mean being ill.”
Mannheim’s research found that healthcare professionals often stand in the way of older people using technology-based treatments due to negative attitudes towards age. “So, actually, a barrier to using these technologies could be that healthcare professionals don’t think that someone can use it or won’t even offer it because someone looks old or is old,” he said.
The Impacts
Discrimination impacts the physical, mental, and social well-being of its victims. This includes attitudes towards age.
The PLOS ONE review of research on the global reach of ageism found that experienced or self-determined ageism was associated with significantly worse health outcomes across all countries examined. The same research team calculated that an estimated 6.3 million cases of depression worldwide are linked to ageism.
Other research has found that exposure to negative age stereotyping impacts willingness to adopt a healthy lifestyle in addition to increasing the risk for cardiovascular events.
What Can Be Done?
“Healthcare professionals frequently interact with older adults at their most vulnerable, which can reinforce negative stereotypes of older people being vulnerable or ill,” said Swift. “However, not all older adults fit these stereotypes. Many can live well and independently. Perhaps healthcare education should include reminders of the diverse experiences of older individuals rather than solely focusing on the moments when they require help.”
Research indicates that although progress has been made in geriatric training and the care of older individuals by healthcare education institutions, improved education and training are still needed at all levels of geriatric healthcare, including hospital administrators, physicians, nurses, personal caregivers, and associated health professions.
“Generally speaking, what healthcare professionals learn about aging tends to focus more on the biological aspects,” said Mannheim. “However, they may not fully understand what it means to be old or how to interact with older individuals, especially regarding technology. It is important to raise awareness about ageism because, in my experience working with healthcare professionals, even a single workshop on ageism can have a profound impact. Participants often respond with surprise, saying something like, ‘Wow, I never thought about this before.’”
Mannheim said that training healthcare providers to understand the aging process better could help to reduce any biases they might have and better prepare them to respond more adequately to the needs of older patients.
“We cannot devalue the lives of older people simply because they are older. It is crucial for all of us, especially governments, to acknowledge our responsibility to protect and promote human rights for individuals of all ages. If we fail to do this, the strategies we’ve witnessed during this pandemic will be repeated in the future,” said Nena Georgantzi, PhD, Barcelona-based human rights manager at AGE Platform Europe, an EU network of organizations of and for older people.
A version of this article appeared on Medscape.com.
People are living longer in Europe. Life expectancy increased on the continent by around 12 years between 1960 and 2022. And despite slower progress during the COVID-19 pandemic, the trend appears to be continuing.
Not only are Europeans living longer, their fertility rates are declining. This means that the number of people aged 75-84 years is projected to grow in Europe a full 56.1% by 2050, while the population younger than 55 years is expected to fall by 13.5%.
This means that attitudes toward age need to change, and fast — even among healthcare professionals.
Healthcare Is Not Exempt From Ageist Attitudes
A systematic review published in the journal PLOS ONE in 2020 found that age was a determinant factor in dictating who received certain medical procedures or treatments. For example, a study of 9105 hospitalized patients found that healthcare providers were significantly more likely to withhold life-sustaining treatments from older patients. Another study found evidence that older people are excluded from clinical trials, even when the trials are for diseases that appear later in life, like Parkinson’s.
“In healthcare, there are different levels of ageism,” explained Hannah Swift, PhD, reader in social and organizational psychology at the University of Kent in the United Kingdom.
Ageism is embedded in the laws, rules, and practices of institutions, she explained. This became especially obvious during the pandemic, when health professionals had to decide who to treat, possibly using age as a proxy for making some of these decisions, she said.
“When you categorize people, you might be using stereotypes, assumptions, and expectations about age and that age group to make those decisions, and that’s where errors can occur.”
She added that ageist attitudes also become apparent at the interpersonal level by using patronizing language or offering unnecessary help to older people based on assumptions about their cognitive and physical abilities.
“Older age is often wrongly associated with declining levels of health and activity,” said Ittay Mannheim, PhD, guest postdoctoral researcher on aging and ageism at the Open University of the Netherlands. “However, older adults are a very diverse group, varying widely in many aspects, including health conditions. This stereotype can influence how healthcare professionals interact with them, assuming frailty or memory issues simply based on age. It’s important to recognize that being older doesn’t necessarily mean being ill.”
Mannheim’s research found that healthcare professionals often stand in the way of older people using technology-based treatments due to negative attitudes towards age. “So, actually, a barrier to using these technologies could be that healthcare professionals don’t think that someone can use it or won’t even offer it because someone looks old or is old,” he said.
The Impacts
Discrimination impacts the physical, mental, and social well-being of its victims. This includes attitudes towards age.
The PLOS ONE review of research on the global reach of ageism found that experienced or self-determined ageism was associated with significantly worse health outcomes across all countries examined. The same research team calculated that an estimated 6.3 million cases of depression worldwide are linked to ageism.
Other research has found that exposure to negative age stereotyping impacts willingness to adopt a healthy lifestyle in addition to increasing the risk for cardiovascular events.
What Can Be Done?
“Healthcare professionals frequently interact with older adults at their most vulnerable, which can reinforce negative stereotypes of older people being vulnerable or ill,” said Swift. “However, not all older adults fit these stereotypes. Many can live well and independently. Perhaps healthcare education should include reminders of the diverse experiences of older individuals rather than solely focusing on the moments when they require help.”
Research indicates that although progress has been made in geriatric training and the care of older individuals by healthcare education institutions, improved education and training are still needed at all levels of geriatric healthcare, including hospital administrators, physicians, nurses, personal caregivers, and associated health professions.
“Generally speaking, what healthcare professionals learn about aging tends to focus more on the biological aspects,” said Mannheim. “However, they may not fully understand what it means to be old or how to interact with older individuals, especially regarding technology. It is important to raise awareness about ageism because, in my experience working with healthcare professionals, even a single workshop on ageism can have a profound impact. Participants often respond with surprise, saying something like, ‘Wow, I never thought about this before.’”
Mannheim said that training healthcare providers to understand the aging process better could help to reduce any biases they might have and better prepare them to respond more adequately to the needs of older patients.
“We cannot devalue the lives of older people simply because they are older. It is crucial for all of us, especially governments, to acknowledge our responsibility to protect and promote human rights for individuals of all ages. If we fail to do this, the strategies we’ve witnessed during this pandemic will be repeated in the future,” said Nena Georgantzi, PhD, Barcelona-based human rights manager at AGE Platform Europe, an EU network of organizations of and for older people.
A version of this article appeared on Medscape.com.
Higher Early-Onset CRC Mortality Seen in Racial, Ethnic Minorities
TOPLINE:
The largest racial and ethnic disparities in survival were linked to neighborhood socioeconomic status.
METHODOLOGY:
- US rates of EOCRC are increasing, with differences across racial and ethnic groups, but few studies have provided detailed risk estimates in the categories of Asian American and of Native Hawaiian or Other Pacific Islander, as well as the contribution of sociodemographic factors to these differences.
- A population-based cohort study analyzed California Cancer Registry data for 22,834 individuals aged 18-49 years diagnosed with EOCRC between January 2000 and December 2019.
- Researchers examined the association between mortality risk and racial and ethnic groups, including Asian American (15.5%, separated into seven subcategories), Hispanic (30.2%), Native Hawaiian or Other Pacific Islander (0.6%), non-Hispanic American Indian or Alaska Native (0.5%), non-Hispanic Black (7.3%), and non-Hispanic White (45.9%) individuals, with a median follow-up of 4.2 years.
- Statistical models measured baseline associations adjusting for clinical features and then tested for the contribution of socioeconomic factors together and separately, with adjustments for insurance status, neighborhood socioeconomic status, and more.
TAKEAWAY:
- Native Hawaiian or Other Pacific Islander individuals demonstrated the highest EOCRC mortality risk compared with non-Hispanic White individuals (socioeconomic status–adjusted HR [SES aHR], 1.34; 95% CI, 1.01-1.76).
- Non-Hispanic Black individuals showed a higher EOCRC mortality risk than non-Hispanic White individuals (SES aHR, 1.18; 95% CI, 1.07-1.29).
- Hispanic individuals’ higher EOCRC mortality (base aHR, 1.15; 95% CI, 1.08-1.22) disappeared after adjusting for neighborhood socioeconomic status (SES aHR, 0.98; 95% CI, 0.92-1.04).
- Southeast Asian individuals’ increased mortality risk (base aHR, 1.17; 95% CI, 1.03-1.34) was no longer significant after adjusting for insurance status (SES aHR, 1.10; 95% CI, 0.96-1.26).
IN PRACTICE:
“As clinicians and researchers, we should ask ourselves how to act on these findings,” wrote the authors of an invited commentary. “The effort cannot stop with data analysis alone, it must extend to actionable steps,” such as tailored efforts to deliver culturally competent care and patient navigation services to those with greatest need and at highest risk, they added.
SOURCE:
The study was led by Joshua Demb, PhD, University of California, San Diego. The study was published online on November 22 in JAMA Network Open (2024. doi: 10.1001/jamanetworkopen.2024.46820) with the invited commentary led by Clare E. Jacobson, MD, University of Michigan, Ann Arbor.
LIMITATIONS:
The study was limited by a relatively short follow-up time and small sample sizes in some racial and ethnic groups, potentially leading to imprecise aHR estimates. The generalizability of findings beyond California requires further investigation, and the ability to examine potential associations between neighborhood socioeconomic status and other factors was also constrained by small sample sizes.
DISCLOSURES:
The study received support from the National Cancer Institute at the National Institutes of Health. One study author reported receiving consulting fees from Guardant Health, InterVenn Biosciences, Geneoscopy, and Universal DX; research support from Freenome; and stock options from CellMax outside the submitted work. No other disclosures were reported by other authors of the study or the commentary.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
The largest racial and ethnic disparities in survival were linked to neighborhood socioeconomic status.
METHODOLOGY:
- US rates of EOCRC are increasing, with differences across racial and ethnic groups, but few studies have provided detailed risk estimates in the categories of Asian American and of Native Hawaiian or Other Pacific Islander, as well as the contribution of sociodemographic factors to these differences.
- A population-based cohort study analyzed California Cancer Registry data for 22,834 individuals aged 18-49 years diagnosed with EOCRC between January 2000 and December 2019.
- Researchers examined the association between mortality risk and racial and ethnic groups, including Asian American (15.5%, separated into seven subcategories), Hispanic (30.2%), Native Hawaiian or Other Pacific Islander (0.6%), non-Hispanic American Indian or Alaska Native (0.5%), non-Hispanic Black (7.3%), and non-Hispanic White (45.9%) individuals, with a median follow-up of 4.2 years.
- Statistical models measured baseline associations adjusting for clinical features and then tested for the contribution of socioeconomic factors together and separately, with adjustments for insurance status, neighborhood socioeconomic status, and more.
TAKEAWAY:
- Native Hawaiian or Other Pacific Islander individuals demonstrated the highest EOCRC mortality risk compared with non-Hispanic White individuals (socioeconomic status–adjusted HR [SES aHR], 1.34; 95% CI, 1.01-1.76).
- Non-Hispanic Black individuals showed a higher EOCRC mortality risk than non-Hispanic White individuals (SES aHR, 1.18; 95% CI, 1.07-1.29).
- Hispanic individuals’ higher EOCRC mortality (base aHR, 1.15; 95% CI, 1.08-1.22) disappeared after adjusting for neighborhood socioeconomic status (SES aHR, 0.98; 95% CI, 0.92-1.04).
- Southeast Asian individuals’ increased mortality risk (base aHR, 1.17; 95% CI, 1.03-1.34) was no longer significant after adjusting for insurance status (SES aHR, 1.10; 95% CI, 0.96-1.26).
IN PRACTICE:
“As clinicians and researchers, we should ask ourselves how to act on these findings,” wrote the authors of an invited commentary. “The effort cannot stop with data analysis alone, it must extend to actionable steps,” such as tailored efforts to deliver culturally competent care and patient navigation services to those with greatest need and at highest risk, they added.
SOURCE:
The study was led by Joshua Demb, PhD, University of California, San Diego. The study was published online on November 22 in JAMA Network Open (2024. doi: 10.1001/jamanetworkopen.2024.46820) with the invited commentary led by Clare E. Jacobson, MD, University of Michigan, Ann Arbor.
LIMITATIONS:
The study was limited by a relatively short follow-up time and small sample sizes in some racial and ethnic groups, potentially leading to imprecise aHR estimates. The generalizability of findings beyond California requires further investigation, and the ability to examine potential associations between neighborhood socioeconomic status and other factors was also constrained by small sample sizes.
DISCLOSURES:
The study received support from the National Cancer Institute at the National Institutes of Health. One study author reported receiving consulting fees from Guardant Health, InterVenn Biosciences, Geneoscopy, and Universal DX; research support from Freenome; and stock options from CellMax outside the submitted work. No other disclosures were reported by other authors of the study or the commentary.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
The largest racial and ethnic disparities in survival were linked to neighborhood socioeconomic status.
METHODOLOGY:
- US rates of EOCRC are increasing, with differences across racial and ethnic groups, but few studies have provided detailed risk estimates in the categories of Asian American and of Native Hawaiian or Other Pacific Islander, as well as the contribution of sociodemographic factors to these differences.
- A population-based cohort study analyzed California Cancer Registry data for 22,834 individuals aged 18-49 years diagnosed with EOCRC between January 2000 and December 2019.
- Researchers examined the association between mortality risk and racial and ethnic groups, including Asian American (15.5%, separated into seven subcategories), Hispanic (30.2%), Native Hawaiian or Other Pacific Islander (0.6%), non-Hispanic American Indian or Alaska Native (0.5%), non-Hispanic Black (7.3%), and non-Hispanic White (45.9%) individuals, with a median follow-up of 4.2 years.
- Statistical models measured baseline associations adjusting for clinical features and then tested for the contribution of socioeconomic factors together and separately, with adjustments for insurance status, neighborhood socioeconomic status, and more.
TAKEAWAY:
- Native Hawaiian or Other Pacific Islander individuals demonstrated the highest EOCRC mortality risk compared with non-Hispanic White individuals (socioeconomic status–adjusted HR [SES aHR], 1.34; 95% CI, 1.01-1.76).
- Non-Hispanic Black individuals showed a higher EOCRC mortality risk than non-Hispanic White individuals (SES aHR, 1.18; 95% CI, 1.07-1.29).
- Hispanic individuals’ higher EOCRC mortality (base aHR, 1.15; 95% CI, 1.08-1.22) disappeared after adjusting for neighborhood socioeconomic status (SES aHR, 0.98; 95% CI, 0.92-1.04).
- Southeast Asian individuals’ increased mortality risk (base aHR, 1.17; 95% CI, 1.03-1.34) was no longer significant after adjusting for insurance status (SES aHR, 1.10; 95% CI, 0.96-1.26).
IN PRACTICE:
“As clinicians and researchers, we should ask ourselves how to act on these findings,” wrote the authors of an invited commentary. “The effort cannot stop with data analysis alone, it must extend to actionable steps,” such as tailored efforts to deliver culturally competent care and patient navigation services to those with greatest need and at highest risk, they added.
SOURCE:
The study was led by Joshua Demb, PhD, University of California, San Diego. The study was published online on November 22 in JAMA Network Open (2024. doi: 10.1001/jamanetworkopen.2024.46820) with the invited commentary led by Clare E. Jacobson, MD, University of Michigan, Ann Arbor.
LIMITATIONS:
The study was limited by a relatively short follow-up time and small sample sizes in some racial and ethnic groups, potentially leading to imprecise aHR estimates. The generalizability of findings beyond California requires further investigation, and the ability to examine potential associations between neighborhood socioeconomic status and other factors was also constrained by small sample sizes.
DISCLOSURES:
The study received support from the National Cancer Institute at the National Institutes of Health. One study author reported receiving consulting fees from Guardant Health, InterVenn Biosciences, Geneoscopy, and Universal DX; research support from Freenome; and stock options from CellMax outside the submitted work. No other disclosures were reported by other authors of the study or the commentary.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Disparities in Skin Cancer Outcomes in the Latine/Hispanic Population
The Latine/Hispanic population in the United States comprises one of the largest and youngest skin of color communities.1,2 In 2020, this group accounted for 19% of all Americans—a percentage expected to increase to more than 25% by 2060.3
It must be emphasized that the Latine/Hispanic community in the United States is incredibly diverse.4 Approximately one-third of individuals in this group are foreign-born, and this community is made up of people from all racialized groups, religions, languages, and cultural identities.2 The heterogeneity of the Latine/Hispanic population translates into a wide representation of skin tones, reflecting a rich range of ancestries, ethnicities, and cultures. The percentage of individuals from each origin group may differ according to where they live in the United States; for instance, individuals who identify as Mexican comprise more than 80% of the Latine/Hispanic population in both Texas and California but only 17% in Florida, where more than half of Latine/Hispanic people identify as Cuban or Puerto Rican.4,5 As a result, when it comes to skin cancer epidemiology, variations in incidence and mortality may exist within each of these subgroups who identify as part of the Latine/Hispanic community, as reported for other cancers.6,7 Further research is needed to investigate these potential differences.Unfortunately, considerable health disparities persist among this rapidly growing population, including increased morbidity and mortality from melanoma and keratinocyte carcinomas (KCs) despite overall low lifetime incidence.8,9 In this review, the epidemiology, clinical manifestation, and ethnic disparities for skin cancer among the US Latine/Hispanic population are summarized; other factors impacting overall health and health care, including sociocultural factors, also are briefly discussed.
Terminology
Before a meaningful dialogue can be had about skin cancer in the Latine/Hispanic population, it is important to contextualize the terms used to identify this patient population, including Latino/Latine and Hispanic. In the early 1970s, the United States adopted the term Hispanic as a way of conglomerating Spanish-speaking individuals from Spain, the Caribbean, and Central and South America. The goal was to implement a common identifier that enabled the US government to study the economic and social development of these groups.10 Nevertheless, considerable differences (eg, variations in skin pigmentation, sun sensitivity) exist among Hispanic communities, with some having stronger European, African, or Amerindian influences due to colonization of their distinct countries.11
In contrast, Latino is a geographic term and refers to people with roots in Latin America and the Caribbean (Table 1).12,13 For example, a person from Brazil may be considered Latino but not Hispanic as Brazilians speak Portuguese; alternatively, Spaniards (who are considered Hispanic) are not Latino because Spain is not a Latin American country. A person from Mexico would be considered both Latino and Hispanic.13
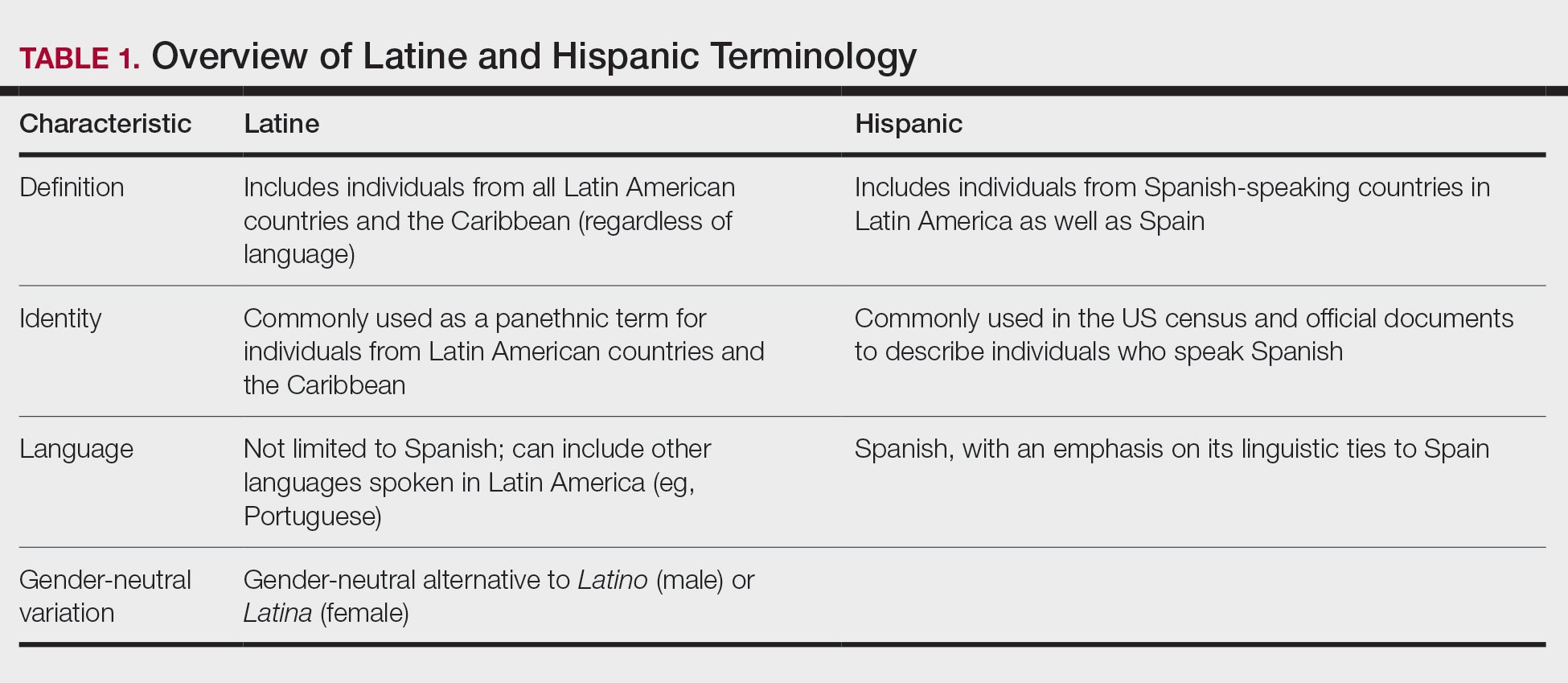
More recently, the term Latine has been introduced as an alternative to the gender binary inherent in the Spanish language.12 For the purposes of this article, the terms Latine and Hispanic will be used interchangeably (unless otherwise specified) depending on how they are cited in the existing literature. Furthermore, the term non-Hispanic White (NHW) will be used to refer to individuals who have been socially ascribed or who self-identify as White in terms of race or ethnicity.
Melanoma
Melanoma, the deadliest form of skin cancer, is more likely to metastasize compared to other forms of skin cancer, including basal cell carcinoma (BCC) and squamous cell carcinoma (SCC). For Latine/Hispanic individuals living in the United States, the lifetime risk for melanoma is 1 in 200 compared to 1 in 33 for NHW individuals.14 While the lifetime risk for melanoma is low for the Latine/Hispanic population, Hispanic individuals are diagnosed with melanoma at an earlier age (mean, 56 years), and the rate of new cases is marginally higher for women (4.9 per 100,000) compared to men (4.8 per 100,000).15,16
Typical sites of melanoma manifestation in Latine/Hispanic individuals include the torso (most common site in Hispanic men), lower extremities (most common site in Hispanic women), and acral sites (palms, soles, and nails).9,16,17 Anatomic location also can vary according to age for both men and women. For men, the incidence of melanoma on the trunk appears to decrease with age, while the incidence on the head and neck may increase. For women, the incidence of melanoma on the lower extremities and hip increases with age. Cutaneous melanoma may manifest as a lesion with asymmetry, irregular borders, variation in pigmentation, large diameter (>6 mm), and evolution over time. In patients with skin of color, melanoma easily can be missed, as it also typically mimics more benign skin conditions and may develop from an existing black- or dark brown–pigmented macule.18 The most common histologic subtype reported among Latine/Hispanic individuals in the United States is superficial spreading melanoma (20%–23%) followed by nodular melanoma and acral lentiginous melanoma.16,19 Until additional risk factors associated with melanoma susceptibility in Hispanic/Latine people are better elucidated, it may be appropriate to use an alternative acronym, such as CUBED (Table 2), in addition to the standard ABCDE system to help recognize potential melanoma on acral sites.18
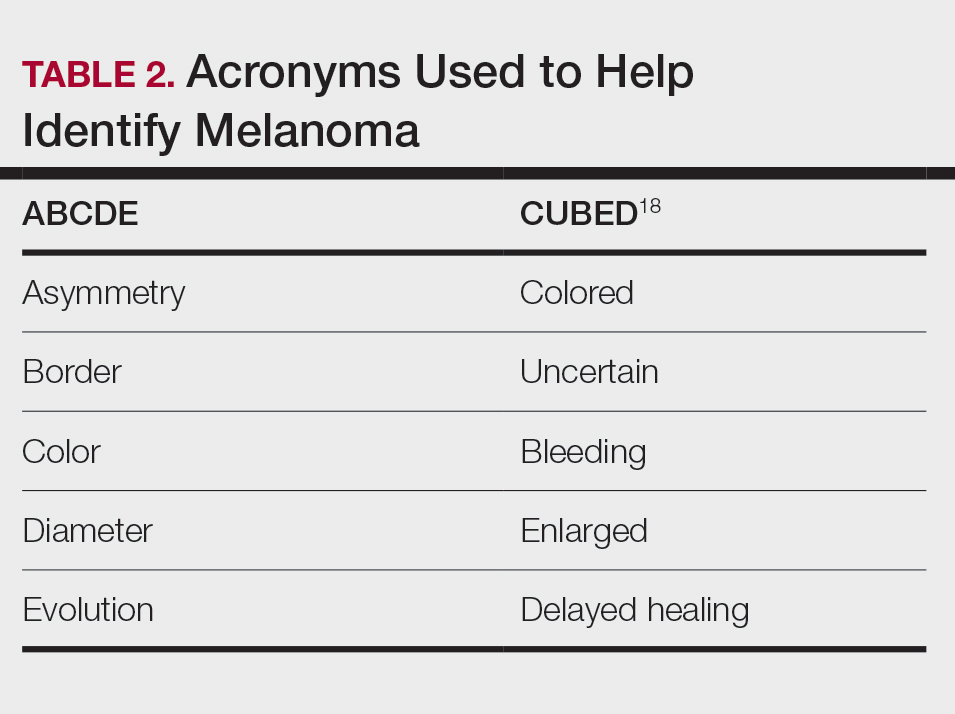
Although the lifetime risk for melanoma among Hispanic individuals in the United States is lower than that for NHW individuals, Hispanic patients who are diagnosed with melanoma are more likely to present with increased tumor thickness and later-stage diagnosis compared to NHW individuals.8,16,20 In a recent study by Qian et al,8 advanced stage melanoma—defined as regional or distant stage disease—was present in 12.6% of NHW individuals. In contrast, the percentage of Hispanics with advanced disease was higher at 21%.8 Even after controlling for insurance and poverty status, Hispanic individuals were at greater risk than NHW individuals for late-stage diagnosis.16,20
Morbidity and mortality also have been shown to be higher in Hispanic patients with cutaneous melanoma.9,17 Reasons for this are multifactorial, with studies specific to melanoma citing challenges associated with early detection in individuals with deeply pigmented skin, a lack of awareness and knowledge about skin cancer among Latine/Hispanic patients, and treatment disparities.21-23 Moreover, very few studies have reported comprehensive data on patients from Africa and Latin America. Studies examining the role of genetic ancestry, epigenetic variants, and skin pigmentation and the risk for melanoma among the Latine/Hispanic population therefore are much needed.24
Keratinocyte Carcinomas
Keratinocyte carcinomas, also known as nonmelanoma skin cancers, include BCC and SCC. In comparison to the high-quality data available for melanoma from cancer registries, there are less reliable incidence data for KCs, especially among individuals with skin of color.25 As a result, KC epidemiology in the United States is drawn largely from case series (especially for individuals with skin of color) or claims data from small data sets often from geographically restricted regions within the United States.25,26
Basal Cell Carcinoma—Basal cell carcinoma is the most common malignant skin cancer in Latine/Hispanic individuals. Among those with lighter skin tones, the lifetime risk for BCC is about 30%.27,28 Men typically are affected at a higher rate than women, and the median age for diagnosis is 68 years.29 The development of BCC primarily is linked to lifetime accumulated UV radiation exposure. Even though BCC has a low mortality rate, it can lead to substantial morbidity due to factors such as tumor location, size, and rate of invasion, resulting in cosmetic and functional issues. Given its low metastatic potential, treatment of BCC typically is aimed at local control.30 Options for treatment include Mohs micrographic surgery (MMS), curettage and electrodessication, cryosurgery, photodynamic therapy, radiation therapy, and topical therapies. Systemic therapies are reserved for patients with locally advanced or metastatic disease.30
Latine/Hispanic patients characteristically present with BCCs on sun-exposed areas of the skin such as the head and neck region. In most patients, BCC manifests as a translucent pearly nodule with superficial telangiectasias and/or a nonhealing ulcer with a central depression and rolled nontender borders. However, in patients with skin of color, 66% of BCCs manifest with pigmentation; in fact, pigmented BCC (a subtype of BCC) has been shown to have a higher prevalence among Hispanic individuals, with an incidence twice as frequent as in NHW individuals.31 In addition, there are reports of increased tendency among Latine/Hispanic individuals to develop multiple BCCs.32,33
The relationship between UV exposure and KCs could explain the relatively higher incidence in populations with skin of color living in warmer climates, including Hispanic individuals.34 Even so, the development of BCCs appears to correlate directly with the degree of pigmentation in the skin, as it is most common in individuals with lighter skin tones within the Hispanic population.25,34,35 Other risk factors associated with BCC development include albinism, arsenic ingestion, chronic infections, immunosuppression, history of radiation treatment, and history of scars or ulcers due to physical/thermal trauma.35-37
Squamous Cell Carcinoma—Squamous cell carcinoma is the second most common skin cancer among Latine/Hispanic patients. In contrast with NHW patients, evidence supporting the role of UV exposure as a primary risk factor for SCC in patients with skin of color remains limited.25,38 Reports linking UV exposure and KCs in Hispanic and Black individuals predominantly include case series or population-based studies that do not consider levels of UV exposure.25
More recently, genetic ancestry analyses of a large multiethnic cohort found an increased risk for cutaneous SCC among Latine/Hispanic individuals with European ancestry compared to those with Native American or African ancestry; however, these genetic ancestry associations were attenuated (although not eliminated) after considering skin pigmentation (using loci associated with skin pigmentation), history of sun exposure (using actinic keratoses as a covariate for chronic sun exposure), and sun-protected vs sun-exposed anatomic sites, supporting the role of other environmental or sociocultural factors in the development of SCC.39 Similar to BCCs, immunosuppression, chronic scarring, skin irritation, and inflammatory disease also are documented risk factors.9,32
Among NHW individuals with lighter skin tones, SCC characteristically manifests on sun-exposed areas of the skin such as the head and neck region. Typically, a lesion may appear as a scaly erythematous papule or plaque that may be verrucous in nature or a nonhealing bleeding ulcer. In patients with more deeply pigmented skin, SCC tends to develop in the perianal region and on the penis and lower legs; pigmented lesions also may be present (as commonly reported in BCCs).9,32,36
Unfortunately, the lower incidence of KCs and lack of surveillance in populations with skin of color result in a low index of clinical suspicion, leading to delayed diagnoses and increased morbidity.40 Keratinocyte carcinomas are more costly to treat and require more health care resources for Latine/Hispanic and Black patients compared to their NHW counterparts; for example, KCs are associated with more ambulatory visits, more prescription medications, and greater cost on a per-person, per-year basis in Latine/Hispanic and Black patients compared with NHW patients.41 Moreover, a recent multicenter retrospective study found Hispanic patients had 17% larger MMS defects following treatment for KCs compared to NHW patients after adjustment for age, sex, and insurance type.42
Hispanic patients tend to present initially with SCCs in areas associated with advanced disease, such as the anogenital region, penis, and the lower extremities. Latine and Black men have the highest incidence of penile SCC, which is rare with high morbidity and mortality.32,43,44 The higher incidence of penile SCC among Hispanic individuals living in southern states could correspond to circumcision or HPV infection rates,44 ultimately impacting incidence.45
Dermatofibrosarcoma Protuberans
Dermatofibrosarcoma protuberans (DFSP) is a rare locally aggressive cutaneous sarcoma. According to population studies, overall incidence of DFSP is around 4.1 to 4.2 per million in the United States. Population-based studies on DFSP are limited, but available data suggest that Black patients as well as women have the highest incidence.46
Dermatofibrosarcoma protuberans is characterized by its capacity to invade surrounding tissues in a tentaclelike pattern.47 This characteristic often leads to inadequate initial resection of the lesion as well as a high recurrence rate despite its low metastatic potential.48 In early stages, DFSP typically manifests as an asymptomatic plaque with a slow growth rate. The color of the lesion ranges from reddish brown to flesh colored. The pigmented form of DFSP, known as Bednar tumor, is the most common among Black patients.47 As the tumor grows, it tends to become firm and nodular. The most common location for
Although current guidelines designate MMS as the first-line treatment for DFSP, the procedure may be inaccessible for certain populations.49 Patients with skin of color are more likely to undergo wide local excision (WLE) than MMS; however, WLE is less effective, with a recurrence rate of 30% compared with 3% in those treated with MMS.50 A retrospective cohort study of more than 2000 patients revealed that Hispanic and Black patients were less likely to undergo MMS. In addition, the authors noted that WLE recipients more commonly were deceased at the end of the study.51
Despite undergoing treatment for a primary DFSP, Hispanic patients also appear to be at increased risk for a second surgery.52 Additional studies are needed to elucidate the reasons behind higher recurrence rates in Latine/Hispanic patients compared to NHW individuals.
Factors Influencing Skin Cancer Outcomes
In recent years, racial and ethnic disparities in health care use, medical treatment, and quality of care among minoritized populations (including Latine/Hispanic groups) have been documented in the medical literature.53,54 These systemic inequities, which are rooted in structural racism,55 have contributed to poorer health outcomes, worse health status, and lower-quality care for minoritized patients living in the United States, including those impacted by dermatologic conditions.8,43,55-57 Becoming familiar with the sociocultural factors influencing skin cancer outcomes in the Latine/Hispanic community (including the lack of or inadequate health insurance, medical mistrust, language, and other cultural elements) and the paucity of research in this domain could help eliminate existing health inequities in this population.
Health Insurance Coverage—Although the uninsured rates in the Latine population have decreased since the passage of the Affordable Care Act (from 30% in 2013 to a low of 19% in 2017),58 inadequate health insurance coverage remains one of the largest barriers to health care access and a contributor to health disparities among the Latine community. Nearly 1 in 5 Latine individuals in the United States are uninsured compared to 8% of NHW individuals.58 Even though Latine individuals are more likely than non-Latine individuals to be part of the workforce, Latine employees are less likely to receive employer-sponsored coverage (27% vs 53% for NHW individuals).59
Not surprisingly, noncitizens are far less likely to be insured; this includes lawfully present immigrants (ie, permanent residents or green card holders, refugees, asylees, and others who are authorized to live in the United States temporarily or permanently) and undocumented immigrants (including individuals who entered the country without authorization and individuals who entered the country lawfully and stayed after their visa or status expired). The higher uninsured rate among noncitizens reflects not only limited access to employer-sponsored coverage but includes immigrant eligibility restrictions for federal programs such as Medicaid, the Children’s Health Insurance Program, and the Affordable Care Act Marketplace coverage.60
With approximately 9 million Americans living in mixed-status families (and nearly 10% of babies born each year with at least one undocumented parent), restrictive federal or state health care policies may extend beyond their stated target and impact both Latine citizens and noncitizens.61-65 For instance, Vargas et al64 found that both Latine citizens and noncitizens who lived in states with a high number of immigration-related laws had decreased odds of reporting optimal health as compared to Latine respondents in states with fewer immigration-related laws. Other barriers to enrollment include fears and confusion about program qualification, even if eligible.58
Medical Mistrust and Unfamiliarity—Mistrust of medical professionals has been shown to reduce patient adherence to treatment as prescribed by their medical provider and can negatively influence health outcomes.53 For racial/ethnic minoritized groups (including Latine/Hispanic patients), medical mistrust may be rooted in patients’ experience of discrimination in the health care setting. In a recent cross-sectional study, results from a survey of California adults (including 704 non-Hispanic Black, 711 Hispanic, and 913 NHW adults) found links between levels of medical mistrust and perceived discrimination based on race/ethnicity and language as well as perceived discrimination due to income level and type or lack of insurance.53 Interestingly, discrimination attributed to income level and insurance status remained after controlling for race/ethnicity and language. As expected, patients reliant on public insurance programs such as Medicare have been reported to have greater medical mistrust and suspicion compared with private insurance holders.65 Together, these findings support the notion that individuals who have low socioeconomic status and lack insurance coverage—disproportionately historically marginalized populations—are more likely to perceive discrimination in health care settings, have greater medical mistrust, and experience poorer health outcomes.53
It also is important for health care providers to consider that the US health care system is unfamiliar to many Latine/Hispanic individuals. Costs of medical services tend to be substantially higher in the United States, which can contribute to mistrust in the system.66 In addition, unethical medical experimentations have negatively affected both Latine and especially non-Hispanic Black populations, with long-lasting perceptions of deception and exploitation.67 These beliefs have undermined the trust that these populations have in clinicians and the health care system.54,67
Language and Other Cultural Elements—The inability to effectively communicate with health care providers could contribute to disparities in access to and use of health care services among Latine/Hispanic individuals. In a Medical Expenditure Panel Survey analysis, half of Hispanic patients with limited comfort speaking English did not have a usual source of care, and almost 90% of those with a usual source of care had a provider who spoke Spanish or used interpreters—indicating that few Hispanic individuals with limited comfort speaking English selected a usual source of care without language assistance.68,69 In other examples, language barriers contributed to disparities in cancer screening, and individuals with limited English proficiency were more likely to have difficulty understanding their physician due to language barriers.68,70
Improving cultural misconceptions regarding skin conditions, especially skin cancer, is another important consideration in the Latine/Hispanic community. Many Latine/Hispanic individuals wrongly believe they cannot develop skin cancer due to their darker skin tones and lack of family history.26 Moreover, multiple studies assessing melanoma knowledge and perception among participants with skin of color (including one with an equal number of Latine/Hispanic, Black/African American, and Asian individuals for a total of 120 participants) revealed that many were unaware of the risk for melanoma on acral sites.71 Participants expressed a need for more culturally relevant content from both clinicians and public materials (eg, images of acral melanoma in a person with skin of color).71-73
Paucity of Research—There is limited research regarding skin cancer risks and methods of prevention for patients with skin of color, including the Latine/Hispanic population. Efforts to engage and include patients from these communities, as well as clinicians or investigators from similar backgrounds, in clinical studies are desperately needed. It also is important that clinical studies collect data beyond population descriptors to account for both clinical and genetic variations observed in the Latine/Hispanic population.
Latine/Hispanic individuals are quite diverse with many variable factors that may influence skin cancer outcomes. Often, cancer surveillance data are available in aggregate only, which could mask this heterogeneity.74 Rigorous studies that collect more granular data, including objective measures of skin pigmentation beyond self-reported Fitzpatrick skin type, culture/beliefs, lifestyle/behavior, geographic location, socioeconomic status, genetics, or epigenetics could help fully elucidate skin cancer risks and mitigate health disparities among individuals who identify as part of this population.
Final Thoughts
The Latine/Hispanic community—the largest ethnic minoritized group in the United States—is disproportionately affected by dermatologic health disparities. We hope this review helps to increase recognition of the clinical manifestations of skin cancer in Latine/Hispanic patients. Other factors that may impact skin cancer outcomes in this population include (but are not limited to) lack of or inadequate health insurance, medical mistrust, linguistic barriers and/or individual/cultural perspectives, along with limited research. Recognizing and addressing these (albeit complex) barriers that contribute to the inequitable access to health care in this population remains a critical step toward improving skin cancer outcomes.
- Noe-Bustamnate L, Lopez MH, Krogstad JM. US Hispanic population surpassed 60 million in 2019, but growth has slowed. July 7, 2020. Accessed September 3, 2024. https://www.pewresearch.org/short-reads/2020/07/07/u-s-hispanic-population-surpassed-60-million-in-2019-but-growth-has-slowed/
- Frank C, Lopez MH. Hispanic Americans’ trust in and engagement with science. Pew Research Center. June 14, 2022. Accessed September 3, 2024. https://www.pewresearch.org/wp-content/uploads/sites/20/2022/06/PS_2022.06.14_hispanic-americans-science_REPORT.pdf
- US Census Bureau. Projections of the size and composition of the US population: 2014 to 2060. US Government Printing Office; 2015. Accessed September 5, 2024. https://www.census.gov/content/dam/Census/library/publications/2015/demo/p25-1143.pdf
- Zong J. A mosaic, not a monolith: a profile of the U.S. Latino population, 2000-2020. October 26, 2022. Accessed September 3, 2024. https://latino.ucla.edu/research/latino-population-2000-2020/
- Latinos in California, Texas, New York, Florida and New Jersey. Pew Research Center. March 19, 2004. Accessed September 3, 2024. https://www.pewresearch.org/hispanic/2004/03/19/latinos-in-california-texas-new-york-florida-and-new-jersey/
- Pinheiro PS, Sherman RL, Trapido EJ, et al. Cancer incidence in first generation US Hispanics: Cubans, Mexicans, Puerto Ricans, and new Latinos. Cancer Epidemiol Biomarkers Prev. 2009;18:2162-2169.
- Pinheiro PS, Callahan KE, Kobetz EN. Disaggregated Hispanic groups and cancer: importance, methodology, and current knowledge. In: Ramirez AG, Trapido EJ, eds. Advancing the Science of Cancer in Latinos. Springer; 2020:17-34.
- Qian Y, Johannet P, Sawyers A, et al. The ongoing racial disparities in melanoma: an analysis of the Surveillance, Epidemiology, and End Results database (1975-2016). J Am Acad Dermatol. 2021;84:1585-1593.
- Hogue L, Harvey VM. Basal cell carcinoma, squamous cell carcinoma, and cutaneous melanoma in skin of color patients. Dermatol Clin. 2019;37:519-526.
- Cruzval-O’Reilly E, Lugo-Somolinos A. Melanoma in Hispanics: we may have it all wrong. Cutis. 2020;106:28-30.
- Borrell LN, Elhawary JR, Fuentes-Afflick E, et al. Race and genetic ancestry in medicine—a time for reckoning with racism. N Engl J Med. 2021;384:474-480.
- Lopez MH, Krogstad JM, Passel JS. Who is Hispanic? September 5, 2023. Accessed September 3, 2024. https://www.pewresearch.org/short-reads/2023/09/05/who-is-hispanic/
- Carrasquillo OY, Lambert J, Merritt BG. Comment on “Disparities in nonmelanoma skin cancer in Hispanic/Latino patients based on Mohs micrographic surgery defect size: a multicenter retrospective study.”J Am Acad Dermatol. 2022;87:E129-E130.
- American Cancer Society. Key statistics for melanoma skin cancer. Updated January 17, 2024. Accessed September 3, 2024. https://www.cancer.org/cancer/types/melanoma-skin-cancer/about/key-statistics.html
- National Cancer Institute. Melanoma of the skin: recent trends in SEER age-adjusted incidence rates, 2000-2021. Updated June 27, 2024. Accessed September 3, 2024. https://seer.cancer.gov/statistics-network/explorer/application.htmlsite=53&data_type=1&graph_type=2&compareBy=sex&chk_sex_3=3&chk_sex_2=2&rate_type=2&race=6&age_range=1&stage=101&advopt_precision=1&advopt_show_ci=on&hdn_view=0&advopt_display=2
- Garnett E, Townsend J, Steele B, et al. Characteristics, rates, and trends of melanoma incidence among Hispanics in the USA. Cancer Causes Control. 2016;27:647-659.
- Higgins S, Nazemi A, Feinstein S, et al. Clinical presentations of melanoma in African Americans, Hispanics, and Asians. Dermatol Surg. 2019;45:791-801.
- Bristow IR, de Berker DA, Acland KM, et al. Clinical guidelines for the recognition of melanoma of the foot and nail unit. J Foot Ankle Res. 2010;3:25.
- Fernandez JM, Mata EM, Behbahani S, et al. Survival of Hispanic patients with cutaneous melanoma: a retrospective cohort analysis of 6016 cases from the National Cancer Database. J Am Acad Dermatol. 2023;88:1135-1138.
- Hu S, Sherman R, Arheart K, et al. Predictors of neighborhood risk for late-stage melanoma: addressing disparities through spatial analysis and area-based measures. J Investigative Dermatol. 2014;134:937-945.
- Buster KJ, You Z, Fouad M, et al. Skin cancer risk perceptions: a comparison across ethnicity, age, education, gender, and income. J Am Acad Dermatol. 2012;66:771-779.
- Halpern MT, Ward EM, Pavluck AL, et al. Association of insurance status and ethnicity with cancer stage at diagnosis for 12 cancer sites: a retrospective analysis. Lancet Oncology. 2008;9:222-231.
- Weiss J, Kirsner RS, Hu S. Trends in primary skin cancer prevention among US Hispanics: a systematic review. J Drugs Dermatol. 2012;11:580-586.
- Carvalho LAD, Aguiar FC, Smalley KSM, et al. Acral melanoma: new insights into the immune and genomic landscape. Neoplasia. 2023;46:100947.
- Kolitz E, Lopes F, Arffa M, et al. UV Exposure and the risk of keratinocyte carcinoma in skin of color: a systematic review. JAMA Dermatol. 2022;158:542-546.
- Lukowiak TM, Aizman L, Perz A, et al. Association of age, sex, race, and geographic region with variation of the ratio of basal cell to cutaneous squamous cell carcinomas in the United States. JAMA Dermatol. 2020;156:1192-1198.
- Basset-Seguin N, Herms F. Update in the management of basal cell carcinoma. Acta Derm Venereol. 2020;100:adv00140.
- McDaniel B, Badri T, Steele RB. Basal cell carcinoma. StatPearls [Internet]. Updated March 13, 2024. Accessed September 3, 2024. https://www.ncbi.nlm.nih.gov/books/NBK482439/
- Dessinioti C, Antoniou C, Katsambas A, et al. Basal cell carcinoma: what’s new under the sun. Photochem Photobiol. 2010;86:481-491.
- Kim DP, Kus KJB, Ruiz E. Basal cell carcinoma review. Hematol Oncol Clin North Am. 2019;33:13-24.
- Bigler C, Feldman J, Hall E, et al. Pigmented basal cell carcinoma in Hispanics. J Am Acad Dermatol. 1996;34(5 pt 1):751-752.
- Higgins S, Nazemi A, Chow M, et al. Review of nonmelanoma skin cancer in African Americans, Hispanics, and Asians. Dermatol Surg. 2018;44:903-910.
- Byrd-Miles K, Toombs EL, Peck GL. Skin cancer in individuals of African, Asian, Latin-American, and American-Indian descent: differences in incidence, clinical presentation, and survival compared to Caucasians. J Drugs Dermatol. 2007;6:10-16.
- Rivas M, Rojas E, Calaf GM, et al. Association between non-melanoma and melanoma skin cancer rates, vitamin D and latitude. Oncol Lett. 2017;13:3787-3792.
- Bradford PT. Skin cancer in skin of color. Dermatol Nurs. 2009;21:170-177, 206.
- Davis DS, Robinson C, Callender VD. Skin cancer in women of color: epidemiology, pathogenesis and clinical manifestations. Int J Womens Dermatol. 2021;7:127-134.
- Maafs E, De la Barreda F, Delgado R, et al. Basal cell carcinoma of trunk and extremities. Int J Dermatol. 1997;36:622-628.
- Munjal A, Ferguson N. Skin cancer in skin of color. Dermatol Clin. 2023;41:481-489.
- Jorgenson E, Choquet H, Yin J, et al. Genetic ancestry, skin pigmentation, and the risk of cutaneous squamous cell carcinoma in Hispanic/Latino and non-Hispanic white populations. Commun Biol. 2020;3:765.
- Soliman YS, Mieczkowska K, Zhu TR, et al. Characterizing basal cell carcinoma in Hispanic individuals undergoing Mohs micrographic surgery: a 7-year retrospective review at an academic institution in the Bronx. Brit J Dermatol. 2022;187:597-599.
- Sierro TJ, Blumenthal LY, Hekmatjah J, et al. Differences in health care resource utilization and costs for keratinocyte carcinoma among racioethnic groups: a population-based study. J Am Acad Dermatol. 2022;86:373-378.
- Blumenthal LY, Arzeno J, Syder N, et al. Disparities in nonmelanoma skin cancer in Hispanic/Latino patients based on Mohs micrographic surgery defect size: a multicenter retrospective study. J Am Acad Dermatol. 2022;86:353-358.
- Slopnick EA, Kim SP, Kiechle JE, et al. Racial disparities differ for African Americans and Hispanics in the diagnosis and treatment of penile cancer. Urology. 2016;96:22-28.
- Goodman MT, Hernandez BY, Shvetsov YB. Demographic and pathologic differences in the incidence of invasive penile cancer in the United States, 1995-2003. Cancer Epidemiol Biomarkers Prev. 2007;16:1833-1839.
- Thompson EL, Rosen BL, Maness SB. Social determinants of health and human papillomavirus vaccination among young adults, National Health Interview Survey 2016. J Community Health. 2019;44:149-158.
- Hao X, Billings SD, Wu F, et al. Dermatofibrosarcoma protuberans: update on the diagnosis and treatment. J Clin Med. 2020;9:1752.
- Mosallaei D, Lee EB, Lobl M, et al. Rare cutaneous malignancies in skin of color. Dermatol Surg. 2022;48:606-612.
- Criscito MC, Martires KJ, Stein JA. Prognostic factors, treatment, and survival in dermatofibrosarcoma protuberans. JAMA Dermatol. 2016;152:1365-1371.
- Orenstein LAV, Nelson MM, Wolner Z, et al. Differences in outpatient dermatology encounter work relative value units and net payments by patient race, sex, and age. JAMA Dermatol. 2021;157:406-412.
- Lowe GC, Onajin O, Baum CL, et al. A comparison of Mohs micrographic surgery and wide local excision for treatment of dermatofibrosarcoma protuberans with long-term follow-up: the Mayo Clinic experience. Dermatol Surg. 2017;43:98-106.
- Moore KJ, Chang MS, Weiss J, et al. Racial and ethnic differences in the surgical treatment of dermatofibrosarcoma protuberans: a retrospective cohort analysis. J Am Acad Dermatol. 2022;87:245-247.
- Trofymenko O, Bordeaux JS, Zeitouni NC. Survival in patients with primary dermatofibrosarcoma protuberans: National Cancer Database analysis. J Am Acad Dermatol. 2018;78:1125-1134.
- Bazargan M, Cobb S, Assari S. Discrimination and medical mistrust in a racially and ethnically diverse sample of California adults. Ann Fam Med. 2021;19:4-15.
- Smedley BD, Stith AY, Nelson AR, eds. Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care. Washington, DC; 2003.
- Bailey ZD, Krieger N, Agenor M, et al. Structural racism and health inequities in the USA: evidence and interventions. Lancet. 2017;389:1453-1463.
- Tackett KJ, Jenkins F, Morrell DS, et al. Structural racism and its influence on the severity of atopic dermatitis in African American children. Pediatric Dermatol. 2020;37:142-146.
- Greif C, Srivastava D, Nijhawan RI. A retrospective cohort study of dermatofibrosarcoma protuberans at a large metropolitan academic center. JAAD Int. 2022;6:104-106.
- Office of the Assistant Secretary for Planning and Evaluation. Health insurance coverage and access to care among Latinos: recent rrends and key challenges (HP-2021-22). October 8, 2021. Accessed September 3, 2024. https://aspe.hhs.gov/reports/health-insurance-coverage-access-care-among-latinos
- Keisler-Starkey K, Bunch LN. Health insurance coverage in the United States: 2020 (Current Population Reports No. P60-274). US Census Bureau; 2021. https://www.census.gov/content/dam/Census/library/publications/2021/demo/p60-274.pdf
- Kaiser Family Foundation. Key facts on health coverage of immigrants. Updated June 26, 2024. Accessed September 3, 2024. https://www.kff.org/racial-equity-and-health-policy/fact-sheet/key-facts-on-health-coverage-of-immigrants/
- Pew Research Center. Unauthorized immigrants: length of residency, patterns of parenthood. Published December 1, 2011. Accessed October 28, 2024. https://www.pewresearch.org/race-and-ethnicity/2011/12/01/unauthorized-immigrants-length-of-residency-patterns-of-parenthood/
- Schneider J, Schmitt M. Understanding the relationship between racial discrimination and mental health among African American adults: a review. SAGE Open. 2015;5:1-10.
- Philbin MM, Flake M, Hatzenbuehler ML, et al. State-level immigration and immigrant-focused policies as drivers of Latino health disparities in the United States. Soc Sci Med. 2018;199:29-38.
- Vargas ED, Sanchez GR, Juarez M. The impact of punitive immigrant laws on the health of Latina/o Populations. Polit Policy. 2017;45:312-337.
- Sutton AL, He J, Edmonds MC, et al. Medical mistrust in Black breast cancer patients: acknowledging the roles of the trustor and the trustee. J Cancer Educ. 2019;34:600-607.
- Jacobs J. An overview of Latin American healthcare systems. Pacific Prime Latin America. July 31, 2023. Accessed September 3, 2024. https://www.pacificprime.lat/blog/an-overview-of-latin-american-healthcare-systems/
- CDC. Unfair and unjust practices and conditions harm Hispanic and Latino people and drive health disparities. May 15, 2024. Accessed September 3, 2024. https://www.cdc.gov/tobacco-health-equity/collection/hispanic-latino-unfair-and-unjust.html
- Hall IJ, Rim SH, Dasari S. Preventive care use among Hispanic adults with limited comfort speaking English: an analysis of the Medical Expenditure Panel Survey data. Prev Med. 2022;159:107042.
- Brach C, Chevarley FM. Demographics and health care access and utilization of limited-English-proficient and English-proficient Hispanics. Agency for Healthcare Research and Quality. February 2008. http://meps.ahrq.gov/mepsweb/data_files/publications//rf28/rf28.pdf
- Berdahl TA, Kirby JB. Patient-provider communication disparities by limited English proficiency (LEP): trends from the US Medical Expenditure Panel Survey, 2006-2015. J General Intern Med. 2019;34:1434-1440.
- Robinson JK, Joshi KM, Ortiz S, et al. Melanoma knowledge, perception, and awareness in ethnic minorities in Chicago: recommendations regarding education. Psychooncology. 2011;20:313-320.
- Robinson JK, Nodal M, Chavez L, et al. Enhancing the relevance of skin self-examination for Latinos. JAMA Dermatol. 2017;153:717-718.
- Buchanan Lunsford N, Berktold J, Holman DM, et al. Skin cancer knowledge, awareness, beliefs and preventive behaviors among black and hispanic men and women. Prev Med Rep. 2018;12:203-209.
- Madrigal JM, Correa-Mendez M, Arias JD, et al. Hispanic, Latino/a, Latinx, Latine: disentangling the identities of Hispanic/Latino Americans. National Cancer Institute Division of Cancer Epidemiology & Genetics. October 20, 2022. Accessed September 3, 2024. https://dceg.cancer.gov/about/diversity-inclusion/inclusivity-minute/2022/disentangling-identities-hispanic-latino-americans
The Latine/Hispanic population in the United States comprises one of the largest and youngest skin of color communities.1,2 In 2020, this group accounted for 19% of all Americans—a percentage expected to increase to more than 25% by 2060.3
It must be emphasized that the Latine/Hispanic community in the United States is incredibly diverse.4 Approximately one-third of individuals in this group are foreign-born, and this community is made up of people from all racialized groups, religions, languages, and cultural identities.2 The heterogeneity of the Latine/Hispanic population translates into a wide representation of skin tones, reflecting a rich range of ancestries, ethnicities, and cultures. The percentage of individuals from each origin group may differ according to where they live in the United States; for instance, individuals who identify as Mexican comprise more than 80% of the Latine/Hispanic population in both Texas and California but only 17% in Florida, where more than half of Latine/Hispanic people identify as Cuban or Puerto Rican.4,5 As a result, when it comes to skin cancer epidemiology, variations in incidence and mortality may exist within each of these subgroups who identify as part of the Latine/Hispanic community, as reported for other cancers.6,7 Further research is needed to investigate these potential differences.Unfortunately, considerable health disparities persist among this rapidly growing population, including increased morbidity and mortality from melanoma and keratinocyte carcinomas (KCs) despite overall low lifetime incidence.8,9 In this review, the epidemiology, clinical manifestation, and ethnic disparities for skin cancer among the US Latine/Hispanic population are summarized; other factors impacting overall health and health care, including sociocultural factors, also are briefly discussed.
Terminology
Before a meaningful dialogue can be had about skin cancer in the Latine/Hispanic population, it is important to contextualize the terms used to identify this patient population, including Latino/Latine and Hispanic. In the early 1970s, the United States adopted the term Hispanic as a way of conglomerating Spanish-speaking individuals from Spain, the Caribbean, and Central and South America. The goal was to implement a common identifier that enabled the US government to study the economic and social development of these groups.10 Nevertheless, considerable differences (eg, variations in skin pigmentation, sun sensitivity) exist among Hispanic communities, with some having stronger European, African, or Amerindian influences due to colonization of their distinct countries.11
In contrast, Latino is a geographic term and refers to people with roots in Latin America and the Caribbean (Table 1).12,13 For example, a person from Brazil may be considered Latino but not Hispanic as Brazilians speak Portuguese; alternatively, Spaniards (who are considered Hispanic) are not Latino because Spain is not a Latin American country. A person from Mexico would be considered both Latino and Hispanic.13

More recently, the term Latine has been introduced as an alternative to the gender binary inherent in the Spanish language.12 For the purposes of this article, the terms Latine and Hispanic will be used interchangeably (unless otherwise specified) depending on how they are cited in the existing literature. Furthermore, the term non-Hispanic White (NHW) will be used to refer to individuals who have been socially ascribed or who self-identify as White in terms of race or ethnicity.
Melanoma
Melanoma, the deadliest form of skin cancer, is more likely to metastasize compared to other forms of skin cancer, including basal cell carcinoma (BCC) and squamous cell carcinoma (SCC). For Latine/Hispanic individuals living in the United States, the lifetime risk for melanoma is 1 in 200 compared to 1 in 33 for NHW individuals.14 While the lifetime risk for melanoma is low for the Latine/Hispanic population, Hispanic individuals are diagnosed with melanoma at an earlier age (mean, 56 years), and the rate of new cases is marginally higher for women (4.9 per 100,000) compared to men (4.8 per 100,000).15,16
Typical sites of melanoma manifestation in Latine/Hispanic individuals include the torso (most common site in Hispanic men), lower extremities (most common site in Hispanic women), and acral sites (palms, soles, and nails).9,16,17 Anatomic location also can vary according to age for both men and women. For men, the incidence of melanoma on the trunk appears to decrease with age, while the incidence on the head and neck may increase. For women, the incidence of melanoma on the lower extremities and hip increases with age. Cutaneous melanoma may manifest as a lesion with asymmetry, irregular borders, variation in pigmentation, large diameter (>6 mm), and evolution over time. In patients with skin of color, melanoma easily can be missed, as it also typically mimics more benign skin conditions and may develop from an existing black- or dark brown–pigmented macule.18 The most common histologic subtype reported among Latine/Hispanic individuals in the United States is superficial spreading melanoma (20%–23%) followed by nodular melanoma and acral lentiginous melanoma.16,19 Until additional risk factors associated with melanoma susceptibility in Hispanic/Latine people are better elucidated, it may be appropriate to use an alternative acronym, such as CUBED (Table 2), in addition to the standard ABCDE system to help recognize potential melanoma on acral sites.18

Although the lifetime risk for melanoma among Hispanic individuals in the United States is lower than that for NHW individuals, Hispanic patients who are diagnosed with melanoma are more likely to present with increased tumor thickness and later-stage diagnosis compared to NHW individuals.8,16,20 In a recent study by Qian et al,8 advanced stage melanoma—defined as regional or distant stage disease—was present in 12.6% of NHW individuals. In contrast, the percentage of Hispanics with advanced disease was higher at 21%.8 Even after controlling for insurance and poverty status, Hispanic individuals were at greater risk than NHW individuals for late-stage diagnosis.16,20
Morbidity and mortality also have been shown to be higher in Hispanic patients with cutaneous melanoma.9,17 Reasons for this are multifactorial, with studies specific to melanoma citing challenges associated with early detection in individuals with deeply pigmented skin, a lack of awareness and knowledge about skin cancer among Latine/Hispanic patients, and treatment disparities.21-23 Moreover, very few studies have reported comprehensive data on patients from Africa and Latin America. Studies examining the role of genetic ancestry, epigenetic variants, and skin pigmentation and the risk for melanoma among the Latine/Hispanic population therefore are much needed.24
Keratinocyte Carcinomas
Keratinocyte carcinomas, also known as nonmelanoma skin cancers, include BCC and SCC. In comparison to the high-quality data available for melanoma from cancer registries, there are less reliable incidence data for KCs, especially among individuals with skin of color.25 As a result, KC epidemiology in the United States is drawn largely from case series (especially for individuals with skin of color) or claims data from small data sets often from geographically restricted regions within the United States.25,26
Basal Cell Carcinoma—Basal cell carcinoma is the most common malignant skin cancer in Latine/Hispanic individuals. Among those with lighter skin tones, the lifetime risk for BCC is about 30%.27,28 Men typically are affected at a higher rate than women, and the median age for diagnosis is 68 years.29 The development of BCC primarily is linked to lifetime accumulated UV radiation exposure. Even though BCC has a low mortality rate, it can lead to substantial morbidity due to factors such as tumor location, size, and rate of invasion, resulting in cosmetic and functional issues. Given its low metastatic potential, treatment of BCC typically is aimed at local control.30 Options for treatment include Mohs micrographic surgery (MMS), curettage and electrodessication, cryosurgery, photodynamic therapy, radiation therapy, and topical therapies. Systemic therapies are reserved for patients with locally advanced or metastatic disease.30
Latine/Hispanic patients characteristically present with BCCs on sun-exposed areas of the skin such as the head and neck region. In most patients, BCC manifests as a translucent pearly nodule with superficial telangiectasias and/or a nonhealing ulcer with a central depression and rolled nontender borders. However, in patients with skin of color, 66% of BCCs manifest with pigmentation; in fact, pigmented BCC (a subtype of BCC) has been shown to have a higher prevalence among Hispanic individuals, with an incidence twice as frequent as in NHW individuals.31 In addition, there are reports of increased tendency among Latine/Hispanic individuals to develop multiple BCCs.32,33
The relationship between UV exposure and KCs could explain the relatively higher incidence in populations with skin of color living in warmer climates, including Hispanic individuals.34 Even so, the development of BCCs appears to correlate directly with the degree of pigmentation in the skin, as it is most common in individuals with lighter skin tones within the Hispanic population.25,34,35 Other risk factors associated with BCC development include albinism, arsenic ingestion, chronic infections, immunosuppression, history of radiation treatment, and history of scars or ulcers due to physical/thermal trauma.35-37
Squamous Cell Carcinoma—Squamous cell carcinoma is the second most common skin cancer among Latine/Hispanic patients. In contrast with NHW patients, evidence supporting the role of UV exposure as a primary risk factor for SCC in patients with skin of color remains limited.25,38 Reports linking UV exposure and KCs in Hispanic and Black individuals predominantly include case series or population-based studies that do not consider levels of UV exposure.25
More recently, genetic ancestry analyses of a large multiethnic cohort found an increased risk for cutaneous SCC among Latine/Hispanic individuals with European ancestry compared to those with Native American or African ancestry; however, these genetic ancestry associations were attenuated (although not eliminated) after considering skin pigmentation (using loci associated with skin pigmentation), history of sun exposure (using actinic keratoses as a covariate for chronic sun exposure), and sun-protected vs sun-exposed anatomic sites, supporting the role of other environmental or sociocultural factors in the development of SCC.39 Similar to BCCs, immunosuppression, chronic scarring, skin irritation, and inflammatory disease also are documented risk factors.9,32
Among NHW individuals with lighter skin tones, SCC characteristically manifests on sun-exposed areas of the skin such as the head and neck region. Typically, a lesion may appear as a scaly erythematous papule or plaque that may be verrucous in nature or a nonhealing bleeding ulcer. In patients with more deeply pigmented skin, SCC tends to develop in the perianal region and on the penis and lower legs; pigmented lesions also may be present (as commonly reported in BCCs).9,32,36
Unfortunately, the lower incidence of KCs and lack of surveillance in populations with skin of color result in a low index of clinical suspicion, leading to delayed diagnoses and increased morbidity.40 Keratinocyte carcinomas are more costly to treat and require more health care resources for Latine/Hispanic and Black patients compared to their NHW counterparts; for example, KCs are associated with more ambulatory visits, more prescription medications, and greater cost on a per-person, per-year basis in Latine/Hispanic and Black patients compared with NHW patients.41 Moreover, a recent multicenter retrospective study found Hispanic patients had 17% larger MMS defects following treatment for KCs compared to NHW patients after adjustment for age, sex, and insurance type.42
Hispanic patients tend to present initially with SCCs in areas associated with advanced disease, such as the anogenital region, penis, and the lower extremities. Latine and Black men have the highest incidence of penile SCC, which is rare with high morbidity and mortality.32,43,44 The higher incidence of penile SCC among Hispanic individuals living in southern states could correspond to circumcision or HPV infection rates,44 ultimately impacting incidence.45
Dermatofibrosarcoma Protuberans
Dermatofibrosarcoma protuberans (DFSP) is a rare locally aggressive cutaneous sarcoma. According to population studies, overall incidence of DFSP is around 4.1 to 4.2 per million in the United States. Population-based studies on DFSP are limited, but available data suggest that Black patients as well as women have the highest incidence.46
Dermatofibrosarcoma protuberans is characterized by its capacity to invade surrounding tissues in a tentaclelike pattern.47 This characteristic often leads to inadequate initial resection of the lesion as well as a high recurrence rate despite its low metastatic potential.48 In early stages, DFSP typically manifests as an asymptomatic plaque with a slow growth rate. The color of the lesion ranges from reddish brown to flesh colored. The pigmented form of DFSP, known as Bednar tumor, is the most common among Black patients.47 As the tumor grows, it tends to become firm and nodular. The most common location for
Although current guidelines designate MMS as the first-line treatment for DFSP, the procedure may be inaccessible for certain populations.49 Patients with skin of color are more likely to undergo wide local excision (WLE) than MMS; however, WLE is less effective, with a recurrence rate of 30% compared with 3% in those treated with MMS.50 A retrospective cohort study of more than 2000 patients revealed that Hispanic and Black patients were less likely to undergo MMS. In addition, the authors noted that WLE recipients more commonly were deceased at the end of the study.51
Despite undergoing treatment for a primary DFSP, Hispanic patients also appear to be at increased risk for a second surgery.52 Additional studies are needed to elucidate the reasons behind higher recurrence rates in Latine/Hispanic patients compared to NHW individuals.
Factors Influencing Skin Cancer Outcomes
In recent years, racial and ethnic disparities in health care use, medical treatment, and quality of care among minoritized populations (including Latine/Hispanic groups) have been documented in the medical literature.53,54 These systemic inequities, which are rooted in structural racism,55 have contributed to poorer health outcomes, worse health status, and lower-quality care for minoritized patients living in the United States, including those impacted by dermatologic conditions.8,43,55-57 Becoming familiar with the sociocultural factors influencing skin cancer outcomes in the Latine/Hispanic community (including the lack of or inadequate health insurance, medical mistrust, language, and other cultural elements) and the paucity of research in this domain could help eliminate existing health inequities in this population.
Health Insurance Coverage—Although the uninsured rates in the Latine population have decreased since the passage of the Affordable Care Act (from 30% in 2013 to a low of 19% in 2017),58 inadequate health insurance coverage remains one of the largest barriers to health care access and a contributor to health disparities among the Latine community. Nearly 1 in 5 Latine individuals in the United States are uninsured compared to 8% of NHW individuals.58 Even though Latine individuals are more likely than non-Latine individuals to be part of the workforce, Latine employees are less likely to receive employer-sponsored coverage (27% vs 53% for NHW individuals).59
Not surprisingly, noncitizens are far less likely to be insured; this includes lawfully present immigrants (ie, permanent residents or green card holders, refugees, asylees, and others who are authorized to live in the United States temporarily or permanently) and undocumented immigrants (including individuals who entered the country without authorization and individuals who entered the country lawfully and stayed after their visa or status expired). The higher uninsured rate among noncitizens reflects not only limited access to employer-sponsored coverage but includes immigrant eligibility restrictions for federal programs such as Medicaid, the Children’s Health Insurance Program, and the Affordable Care Act Marketplace coverage.60
With approximately 9 million Americans living in mixed-status families (and nearly 10% of babies born each year with at least one undocumented parent), restrictive federal or state health care policies may extend beyond their stated target and impact both Latine citizens and noncitizens.61-65 For instance, Vargas et al64 found that both Latine citizens and noncitizens who lived in states with a high number of immigration-related laws had decreased odds of reporting optimal health as compared to Latine respondents in states with fewer immigration-related laws. Other barriers to enrollment include fears and confusion about program qualification, even if eligible.58
Medical Mistrust and Unfamiliarity—Mistrust of medical professionals has been shown to reduce patient adherence to treatment as prescribed by their medical provider and can negatively influence health outcomes.53 For racial/ethnic minoritized groups (including Latine/Hispanic patients), medical mistrust may be rooted in patients’ experience of discrimination in the health care setting. In a recent cross-sectional study, results from a survey of California adults (including 704 non-Hispanic Black, 711 Hispanic, and 913 NHW adults) found links between levels of medical mistrust and perceived discrimination based on race/ethnicity and language as well as perceived discrimination due to income level and type or lack of insurance.53 Interestingly, discrimination attributed to income level and insurance status remained after controlling for race/ethnicity and language. As expected, patients reliant on public insurance programs such as Medicare have been reported to have greater medical mistrust and suspicion compared with private insurance holders.65 Together, these findings support the notion that individuals who have low socioeconomic status and lack insurance coverage—disproportionately historically marginalized populations—are more likely to perceive discrimination in health care settings, have greater medical mistrust, and experience poorer health outcomes.53
It also is important for health care providers to consider that the US health care system is unfamiliar to many Latine/Hispanic individuals. Costs of medical services tend to be substantially higher in the United States, which can contribute to mistrust in the system.66 In addition, unethical medical experimentations have negatively affected both Latine and especially non-Hispanic Black populations, with long-lasting perceptions of deception and exploitation.67 These beliefs have undermined the trust that these populations have in clinicians and the health care system.54,67
Language and Other Cultural Elements—The inability to effectively communicate with health care providers could contribute to disparities in access to and use of health care services among Latine/Hispanic individuals. In a Medical Expenditure Panel Survey analysis, half of Hispanic patients with limited comfort speaking English did not have a usual source of care, and almost 90% of those with a usual source of care had a provider who spoke Spanish or used interpreters—indicating that few Hispanic individuals with limited comfort speaking English selected a usual source of care without language assistance.68,69 In other examples, language barriers contributed to disparities in cancer screening, and individuals with limited English proficiency were more likely to have difficulty understanding their physician due to language barriers.68,70
Improving cultural misconceptions regarding skin conditions, especially skin cancer, is another important consideration in the Latine/Hispanic community. Many Latine/Hispanic individuals wrongly believe they cannot develop skin cancer due to their darker skin tones and lack of family history.26 Moreover, multiple studies assessing melanoma knowledge and perception among participants with skin of color (including one with an equal number of Latine/Hispanic, Black/African American, and Asian individuals for a total of 120 participants) revealed that many were unaware of the risk for melanoma on acral sites.71 Participants expressed a need for more culturally relevant content from both clinicians and public materials (eg, images of acral melanoma in a person with skin of color).71-73
Paucity of Research—There is limited research regarding skin cancer risks and methods of prevention for patients with skin of color, including the Latine/Hispanic population. Efforts to engage and include patients from these communities, as well as clinicians or investigators from similar backgrounds, in clinical studies are desperately needed. It also is important that clinical studies collect data beyond population descriptors to account for both clinical and genetic variations observed in the Latine/Hispanic population.
Latine/Hispanic individuals are quite diverse with many variable factors that may influence skin cancer outcomes. Often, cancer surveillance data are available in aggregate only, which could mask this heterogeneity.74 Rigorous studies that collect more granular data, including objective measures of skin pigmentation beyond self-reported Fitzpatrick skin type, culture/beliefs, lifestyle/behavior, geographic location, socioeconomic status, genetics, or epigenetics could help fully elucidate skin cancer risks and mitigate health disparities among individuals who identify as part of this population.
Final Thoughts
The Latine/Hispanic community—the largest ethnic minoritized group in the United States—is disproportionately affected by dermatologic health disparities. We hope this review helps to increase recognition of the clinical manifestations of skin cancer in Latine/Hispanic patients. Other factors that may impact skin cancer outcomes in this population include (but are not limited to) lack of or inadequate health insurance, medical mistrust, linguistic barriers and/or individual/cultural perspectives, along with limited research. Recognizing and addressing these (albeit complex) barriers that contribute to the inequitable access to health care in this population remains a critical step toward improving skin cancer outcomes.
The Latine/Hispanic population in the United States comprises one of the largest and youngest skin of color communities.1,2 In 2020, this group accounted for 19% of all Americans—a percentage expected to increase to more than 25% by 2060.3
It must be emphasized that the Latine/Hispanic community in the United States is incredibly diverse.4 Approximately one-third of individuals in this group are foreign-born, and this community is made up of people from all racialized groups, religions, languages, and cultural identities.2 The heterogeneity of the Latine/Hispanic population translates into a wide representation of skin tones, reflecting a rich range of ancestries, ethnicities, and cultures. The percentage of individuals from each origin group may differ according to where they live in the United States; for instance, individuals who identify as Mexican comprise more than 80% of the Latine/Hispanic population in both Texas and California but only 17% in Florida, where more than half of Latine/Hispanic people identify as Cuban or Puerto Rican.4,5 As a result, when it comes to skin cancer epidemiology, variations in incidence and mortality may exist within each of these subgroups who identify as part of the Latine/Hispanic community, as reported for other cancers.6,7 Further research is needed to investigate these potential differences.Unfortunately, considerable health disparities persist among this rapidly growing population, including increased morbidity and mortality from melanoma and keratinocyte carcinomas (KCs) despite overall low lifetime incidence.8,9 In this review, the epidemiology, clinical manifestation, and ethnic disparities for skin cancer among the US Latine/Hispanic population are summarized; other factors impacting overall health and health care, including sociocultural factors, also are briefly discussed.
Terminology
Before a meaningful dialogue can be had about skin cancer in the Latine/Hispanic population, it is important to contextualize the terms used to identify this patient population, including Latino/Latine and Hispanic. In the early 1970s, the United States adopted the term Hispanic as a way of conglomerating Spanish-speaking individuals from Spain, the Caribbean, and Central and South America. The goal was to implement a common identifier that enabled the US government to study the economic and social development of these groups.10 Nevertheless, considerable differences (eg, variations in skin pigmentation, sun sensitivity) exist among Hispanic communities, with some having stronger European, African, or Amerindian influences due to colonization of their distinct countries.11
In contrast, Latino is a geographic term and refers to people with roots in Latin America and the Caribbean (Table 1).12,13 For example, a person from Brazil may be considered Latino but not Hispanic as Brazilians speak Portuguese; alternatively, Spaniards (who are considered Hispanic) are not Latino because Spain is not a Latin American country. A person from Mexico would be considered both Latino and Hispanic.13

More recently, the term Latine has been introduced as an alternative to the gender binary inherent in the Spanish language.12 For the purposes of this article, the terms Latine and Hispanic will be used interchangeably (unless otherwise specified) depending on how they are cited in the existing literature. Furthermore, the term non-Hispanic White (NHW) will be used to refer to individuals who have been socially ascribed or who self-identify as White in terms of race or ethnicity.
Melanoma
Melanoma, the deadliest form of skin cancer, is more likely to metastasize compared to other forms of skin cancer, including basal cell carcinoma (BCC) and squamous cell carcinoma (SCC). For Latine/Hispanic individuals living in the United States, the lifetime risk for melanoma is 1 in 200 compared to 1 in 33 for NHW individuals.14 While the lifetime risk for melanoma is low for the Latine/Hispanic population, Hispanic individuals are diagnosed with melanoma at an earlier age (mean, 56 years), and the rate of new cases is marginally higher for women (4.9 per 100,000) compared to men (4.8 per 100,000).15,16
Typical sites of melanoma manifestation in Latine/Hispanic individuals include the torso (most common site in Hispanic men), lower extremities (most common site in Hispanic women), and acral sites (palms, soles, and nails).9,16,17 Anatomic location also can vary according to age for both men and women. For men, the incidence of melanoma on the trunk appears to decrease with age, while the incidence on the head and neck may increase. For women, the incidence of melanoma on the lower extremities and hip increases with age. Cutaneous melanoma may manifest as a lesion with asymmetry, irregular borders, variation in pigmentation, large diameter (>6 mm), and evolution over time. In patients with skin of color, melanoma easily can be missed, as it also typically mimics more benign skin conditions and may develop from an existing black- or dark brown–pigmented macule.18 The most common histologic subtype reported among Latine/Hispanic individuals in the United States is superficial spreading melanoma (20%–23%) followed by nodular melanoma and acral lentiginous melanoma.16,19 Until additional risk factors associated with melanoma susceptibility in Hispanic/Latine people are better elucidated, it may be appropriate to use an alternative acronym, such as CUBED (Table 2), in addition to the standard ABCDE system to help recognize potential melanoma on acral sites.18

Although the lifetime risk for melanoma among Hispanic individuals in the United States is lower than that for NHW individuals, Hispanic patients who are diagnosed with melanoma are more likely to present with increased tumor thickness and later-stage diagnosis compared to NHW individuals.8,16,20 In a recent study by Qian et al,8 advanced stage melanoma—defined as regional or distant stage disease—was present in 12.6% of NHW individuals. In contrast, the percentage of Hispanics with advanced disease was higher at 21%.8 Even after controlling for insurance and poverty status, Hispanic individuals were at greater risk than NHW individuals for late-stage diagnosis.16,20
Morbidity and mortality also have been shown to be higher in Hispanic patients with cutaneous melanoma.9,17 Reasons for this are multifactorial, with studies specific to melanoma citing challenges associated with early detection in individuals with deeply pigmented skin, a lack of awareness and knowledge about skin cancer among Latine/Hispanic patients, and treatment disparities.21-23 Moreover, very few studies have reported comprehensive data on patients from Africa and Latin America. Studies examining the role of genetic ancestry, epigenetic variants, and skin pigmentation and the risk for melanoma among the Latine/Hispanic population therefore are much needed.24
Keratinocyte Carcinomas
Keratinocyte carcinomas, also known as nonmelanoma skin cancers, include BCC and SCC. In comparison to the high-quality data available for melanoma from cancer registries, there are less reliable incidence data for KCs, especially among individuals with skin of color.25 As a result, KC epidemiology in the United States is drawn largely from case series (especially for individuals with skin of color) or claims data from small data sets often from geographically restricted regions within the United States.25,26
Basal Cell Carcinoma—Basal cell carcinoma is the most common malignant skin cancer in Latine/Hispanic individuals. Among those with lighter skin tones, the lifetime risk for BCC is about 30%.27,28 Men typically are affected at a higher rate than women, and the median age for diagnosis is 68 years.29 The development of BCC primarily is linked to lifetime accumulated UV radiation exposure. Even though BCC has a low mortality rate, it can lead to substantial morbidity due to factors such as tumor location, size, and rate of invasion, resulting in cosmetic and functional issues. Given its low metastatic potential, treatment of BCC typically is aimed at local control.30 Options for treatment include Mohs micrographic surgery (MMS), curettage and electrodessication, cryosurgery, photodynamic therapy, radiation therapy, and topical therapies. Systemic therapies are reserved for patients with locally advanced or metastatic disease.30
Latine/Hispanic patients characteristically present with BCCs on sun-exposed areas of the skin such as the head and neck region. In most patients, BCC manifests as a translucent pearly nodule with superficial telangiectasias and/or a nonhealing ulcer with a central depression and rolled nontender borders. However, in patients with skin of color, 66% of BCCs manifest with pigmentation; in fact, pigmented BCC (a subtype of BCC) has been shown to have a higher prevalence among Hispanic individuals, with an incidence twice as frequent as in NHW individuals.31 In addition, there are reports of increased tendency among Latine/Hispanic individuals to develop multiple BCCs.32,33
The relationship between UV exposure and KCs could explain the relatively higher incidence in populations with skin of color living in warmer climates, including Hispanic individuals.34 Even so, the development of BCCs appears to correlate directly with the degree of pigmentation in the skin, as it is most common in individuals with lighter skin tones within the Hispanic population.25,34,35 Other risk factors associated with BCC development include albinism, arsenic ingestion, chronic infections, immunosuppression, history of radiation treatment, and history of scars or ulcers due to physical/thermal trauma.35-37
Squamous Cell Carcinoma—Squamous cell carcinoma is the second most common skin cancer among Latine/Hispanic patients. In contrast with NHW patients, evidence supporting the role of UV exposure as a primary risk factor for SCC in patients with skin of color remains limited.25,38 Reports linking UV exposure and KCs in Hispanic and Black individuals predominantly include case series or population-based studies that do not consider levels of UV exposure.25
More recently, genetic ancestry analyses of a large multiethnic cohort found an increased risk for cutaneous SCC among Latine/Hispanic individuals with European ancestry compared to those with Native American or African ancestry; however, these genetic ancestry associations were attenuated (although not eliminated) after considering skin pigmentation (using loci associated with skin pigmentation), history of sun exposure (using actinic keratoses as a covariate for chronic sun exposure), and sun-protected vs sun-exposed anatomic sites, supporting the role of other environmental or sociocultural factors in the development of SCC.39 Similar to BCCs, immunosuppression, chronic scarring, skin irritation, and inflammatory disease also are documented risk factors.9,32
Among NHW individuals with lighter skin tones, SCC characteristically manifests on sun-exposed areas of the skin such as the head and neck region. Typically, a lesion may appear as a scaly erythematous papule or plaque that may be verrucous in nature or a nonhealing bleeding ulcer. In patients with more deeply pigmented skin, SCC tends to develop in the perianal region and on the penis and lower legs; pigmented lesions also may be present (as commonly reported in BCCs).9,32,36
Unfortunately, the lower incidence of KCs and lack of surveillance in populations with skin of color result in a low index of clinical suspicion, leading to delayed diagnoses and increased morbidity.40 Keratinocyte carcinomas are more costly to treat and require more health care resources for Latine/Hispanic and Black patients compared to their NHW counterparts; for example, KCs are associated with more ambulatory visits, more prescription medications, and greater cost on a per-person, per-year basis in Latine/Hispanic and Black patients compared with NHW patients.41 Moreover, a recent multicenter retrospective study found Hispanic patients had 17% larger MMS defects following treatment for KCs compared to NHW patients after adjustment for age, sex, and insurance type.42
Hispanic patients tend to present initially with SCCs in areas associated with advanced disease, such as the anogenital region, penis, and the lower extremities. Latine and Black men have the highest incidence of penile SCC, which is rare with high morbidity and mortality.32,43,44 The higher incidence of penile SCC among Hispanic individuals living in southern states could correspond to circumcision or HPV infection rates,44 ultimately impacting incidence.45
Dermatofibrosarcoma Protuberans
Dermatofibrosarcoma protuberans (DFSP) is a rare locally aggressive cutaneous sarcoma. According to population studies, overall incidence of DFSP is around 4.1 to 4.2 per million in the United States. Population-based studies on DFSP are limited, but available data suggest that Black patients as well as women have the highest incidence.46
Dermatofibrosarcoma protuberans is characterized by its capacity to invade surrounding tissues in a tentaclelike pattern.47 This characteristic often leads to inadequate initial resection of the lesion as well as a high recurrence rate despite its low metastatic potential.48 In early stages, DFSP typically manifests as an asymptomatic plaque with a slow growth rate. The color of the lesion ranges from reddish brown to flesh colored. The pigmented form of DFSP, known as Bednar tumor, is the most common among Black patients.47 As the tumor grows, it tends to become firm and nodular. The most common location for
Although current guidelines designate MMS as the first-line treatment for DFSP, the procedure may be inaccessible for certain populations.49 Patients with skin of color are more likely to undergo wide local excision (WLE) than MMS; however, WLE is less effective, with a recurrence rate of 30% compared with 3% in those treated with MMS.50 A retrospective cohort study of more than 2000 patients revealed that Hispanic and Black patients were less likely to undergo MMS. In addition, the authors noted that WLE recipients more commonly were deceased at the end of the study.51
Despite undergoing treatment for a primary DFSP, Hispanic patients also appear to be at increased risk for a second surgery.52 Additional studies are needed to elucidate the reasons behind higher recurrence rates in Latine/Hispanic patients compared to NHW individuals.
Factors Influencing Skin Cancer Outcomes
In recent years, racial and ethnic disparities in health care use, medical treatment, and quality of care among minoritized populations (including Latine/Hispanic groups) have been documented in the medical literature.53,54 These systemic inequities, which are rooted in structural racism,55 have contributed to poorer health outcomes, worse health status, and lower-quality care for minoritized patients living in the United States, including those impacted by dermatologic conditions.8,43,55-57 Becoming familiar with the sociocultural factors influencing skin cancer outcomes in the Latine/Hispanic community (including the lack of or inadequate health insurance, medical mistrust, language, and other cultural elements) and the paucity of research in this domain could help eliminate existing health inequities in this population.
Health Insurance Coverage—Although the uninsured rates in the Latine population have decreased since the passage of the Affordable Care Act (from 30% in 2013 to a low of 19% in 2017),58 inadequate health insurance coverage remains one of the largest barriers to health care access and a contributor to health disparities among the Latine community. Nearly 1 in 5 Latine individuals in the United States are uninsured compared to 8% of NHW individuals.58 Even though Latine individuals are more likely than non-Latine individuals to be part of the workforce, Latine employees are less likely to receive employer-sponsored coverage (27% vs 53% for NHW individuals).59
Not surprisingly, noncitizens are far less likely to be insured; this includes lawfully present immigrants (ie, permanent residents or green card holders, refugees, asylees, and others who are authorized to live in the United States temporarily or permanently) and undocumented immigrants (including individuals who entered the country without authorization and individuals who entered the country lawfully and stayed after their visa or status expired). The higher uninsured rate among noncitizens reflects not only limited access to employer-sponsored coverage but includes immigrant eligibility restrictions for federal programs such as Medicaid, the Children’s Health Insurance Program, and the Affordable Care Act Marketplace coverage.60
With approximately 9 million Americans living in mixed-status families (and nearly 10% of babies born each year with at least one undocumented parent), restrictive federal or state health care policies may extend beyond their stated target and impact both Latine citizens and noncitizens.61-65 For instance, Vargas et al64 found that both Latine citizens and noncitizens who lived in states with a high number of immigration-related laws had decreased odds of reporting optimal health as compared to Latine respondents in states with fewer immigration-related laws. Other barriers to enrollment include fears and confusion about program qualification, even if eligible.58
Medical Mistrust and Unfamiliarity—Mistrust of medical professionals has been shown to reduce patient adherence to treatment as prescribed by their medical provider and can negatively influence health outcomes.53 For racial/ethnic minoritized groups (including Latine/Hispanic patients), medical mistrust may be rooted in patients’ experience of discrimination in the health care setting. In a recent cross-sectional study, results from a survey of California adults (including 704 non-Hispanic Black, 711 Hispanic, and 913 NHW adults) found links between levels of medical mistrust and perceived discrimination based on race/ethnicity and language as well as perceived discrimination due to income level and type or lack of insurance.53 Interestingly, discrimination attributed to income level and insurance status remained after controlling for race/ethnicity and language. As expected, patients reliant on public insurance programs such as Medicare have been reported to have greater medical mistrust and suspicion compared with private insurance holders.65 Together, these findings support the notion that individuals who have low socioeconomic status and lack insurance coverage—disproportionately historically marginalized populations—are more likely to perceive discrimination in health care settings, have greater medical mistrust, and experience poorer health outcomes.53
It also is important for health care providers to consider that the US health care system is unfamiliar to many Latine/Hispanic individuals. Costs of medical services tend to be substantially higher in the United States, which can contribute to mistrust in the system.66 In addition, unethical medical experimentations have negatively affected both Latine and especially non-Hispanic Black populations, with long-lasting perceptions of deception and exploitation.67 These beliefs have undermined the trust that these populations have in clinicians and the health care system.54,67
Language and Other Cultural Elements—The inability to effectively communicate with health care providers could contribute to disparities in access to and use of health care services among Latine/Hispanic individuals. In a Medical Expenditure Panel Survey analysis, half of Hispanic patients with limited comfort speaking English did not have a usual source of care, and almost 90% of those with a usual source of care had a provider who spoke Spanish or used interpreters—indicating that few Hispanic individuals with limited comfort speaking English selected a usual source of care without language assistance.68,69 In other examples, language barriers contributed to disparities in cancer screening, and individuals with limited English proficiency were more likely to have difficulty understanding their physician due to language barriers.68,70
Improving cultural misconceptions regarding skin conditions, especially skin cancer, is another important consideration in the Latine/Hispanic community. Many Latine/Hispanic individuals wrongly believe they cannot develop skin cancer due to their darker skin tones and lack of family history.26 Moreover, multiple studies assessing melanoma knowledge and perception among participants with skin of color (including one with an equal number of Latine/Hispanic, Black/African American, and Asian individuals for a total of 120 participants) revealed that many were unaware of the risk for melanoma on acral sites.71 Participants expressed a need for more culturally relevant content from both clinicians and public materials (eg, images of acral melanoma in a person with skin of color).71-73
Paucity of Research—There is limited research regarding skin cancer risks and methods of prevention for patients with skin of color, including the Latine/Hispanic population. Efforts to engage and include patients from these communities, as well as clinicians or investigators from similar backgrounds, in clinical studies are desperately needed. It also is important that clinical studies collect data beyond population descriptors to account for both clinical and genetic variations observed in the Latine/Hispanic population.
Latine/Hispanic individuals are quite diverse with many variable factors that may influence skin cancer outcomes. Often, cancer surveillance data are available in aggregate only, which could mask this heterogeneity.74 Rigorous studies that collect more granular data, including objective measures of skin pigmentation beyond self-reported Fitzpatrick skin type, culture/beliefs, lifestyle/behavior, geographic location, socioeconomic status, genetics, or epigenetics could help fully elucidate skin cancer risks and mitigate health disparities among individuals who identify as part of this population.
Final Thoughts
The Latine/Hispanic community—the largest ethnic minoritized group in the United States—is disproportionately affected by dermatologic health disparities. We hope this review helps to increase recognition of the clinical manifestations of skin cancer in Latine/Hispanic patients. Other factors that may impact skin cancer outcomes in this population include (but are not limited to) lack of or inadequate health insurance, medical mistrust, linguistic barriers and/or individual/cultural perspectives, along with limited research. Recognizing and addressing these (albeit complex) barriers that contribute to the inequitable access to health care in this population remains a critical step toward improving skin cancer outcomes.
- Noe-Bustamnate L, Lopez MH, Krogstad JM. US Hispanic population surpassed 60 million in 2019, but growth has slowed. July 7, 2020. Accessed September 3, 2024. https://www.pewresearch.org/short-reads/2020/07/07/u-s-hispanic-population-surpassed-60-million-in-2019-but-growth-has-slowed/
- Frank C, Lopez MH. Hispanic Americans’ trust in and engagement with science. Pew Research Center. June 14, 2022. Accessed September 3, 2024. https://www.pewresearch.org/wp-content/uploads/sites/20/2022/06/PS_2022.06.14_hispanic-americans-science_REPORT.pdf
- US Census Bureau. Projections of the size and composition of the US population: 2014 to 2060. US Government Printing Office; 2015. Accessed September 5, 2024. https://www.census.gov/content/dam/Census/library/publications/2015/demo/p25-1143.pdf
- Zong J. A mosaic, not a monolith: a profile of the U.S. Latino population, 2000-2020. October 26, 2022. Accessed September 3, 2024. https://latino.ucla.edu/research/latino-population-2000-2020/
- Latinos in California, Texas, New York, Florida and New Jersey. Pew Research Center. March 19, 2004. Accessed September 3, 2024. https://www.pewresearch.org/hispanic/2004/03/19/latinos-in-california-texas-new-york-florida-and-new-jersey/
- Pinheiro PS, Sherman RL, Trapido EJ, et al. Cancer incidence in first generation US Hispanics: Cubans, Mexicans, Puerto Ricans, and new Latinos. Cancer Epidemiol Biomarkers Prev. 2009;18:2162-2169.
- Pinheiro PS, Callahan KE, Kobetz EN. Disaggregated Hispanic groups and cancer: importance, methodology, and current knowledge. In: Ramirez AG, Trapido EJ, eds. Advancing the Science of Cancer in Latinos. Springer; 2020:17-34.
- Qian Y, Johannet P, Sawyers A, et al. The ongoing racial disparities in melanoma: an analysis of the Surveillance, Epidemiology, and End Results database (1975-2016). J Am Acad Dermatol. 2021;84:1585-1593.
- Hogue L, Harvey VM. Basal cell carcinoma, squamous cell carcinoma, and cutaneous melanoma in skin of color patients. Dermatol Clin. 2019;37:519-526.
- Cruzval-O’Reilly E, Lugo-Somolinos A. Melanoma in Hispanics: we may have it all wrong. Cutis. 2020;106:28-30.
- Borrell LN, Elhawary JR, Fuentes-Afflick E, et al. Race and genetic ancestry in medicine—a time for reckoning with racism. N Engl J Med. 2021;384:474-480.
- Lopez MH, Krogstad JM, Passel JS. Who is Hispanic? September 5, 2023. Accessed September 3, 2024. https://www.pewresearch.org/short-reads/2023/09/05/who-is-hispanic/
- Carrasquillo OY, Lambert J, Merritt BG. Comment on “Disparities in nonmelanoma skin cancer in Hispanic/Latino patients based on Mohs micrographic surgery defect size: a multicenter retrospective study.”J Am Acad Dermatol. 2022;87:E129-E130.
- American Cancer Society. Key statistics for melanoma skin cancer. Updated January 17, 2024. Accessed September 3, 2024. https://www.cancer.org/cancer/types/melanoma-skin-cancer/about/key-statistics.html
- National Cancer Institute. Melanoma of the skin: recent trends in SEER age-adjusted incidence rates, 2000-2021. Updated June 27, 2024. Accessed September 3, 2024. https://seer.cancer.gov/statistics-network/explorer/application.htmlsite=53&data_type=1&graph_type=2&compareBy=sex&chk_sex_3=3&chk_sex_2=2&rate_type=2&race=6&age_range=1&stage=101&advopt_precision=1&advopt_show_ci=on&hdn_view=0&advopt_display=2
- Garnett E, Townsend J, Steele B, et al. Characteristics, rates, and trends of melanoma incidence among Hispanics in the USA. Cancer Causes Control. 2016;27:647-659.
- Higgins S, Nazemi A, Feinstein S, et al. Clinical presentations of melanoma in African Americans, Hispanics, and Asians. Dermatol Surg. 2019;45:791-801.
- Bristow IR, de Berker DA, Acland KM, et al. Clinical guidelines for the recognition of melanoma of the foot and nail unit. J Foot Ankle Res. 2010;3:25.
- Fernandez JM, Mata EM, Behbahani S, et al. Survival of Hispanic patients with cutaneous melanoma: a retrospective cohort analysis of 6016 cases from the National Cancer Database. J Am Acad Dermatol. 2023;88:1135-1138.
- Hu S, Sherman R, Arheart K, et al. Predictors of neighborhood risk for late-stage melanoma: addressing disparities through spatial analysis and area-based measures. J Investigative Dermatol. 2014;134:937-945.
- Buster KJ, You Z, Fouad M, et al. Skin cancer risk perceptions: a comparison across ethnicity, age, education, gender, and income. J Am Acad Dermatol. 2012;66:771-779.
- Halpern MT, Ward EM, Pavluck AL, et al. Association of insurance status and ethnicity with cancer stage at diagnosis for 12 cancer sites: a retrospective analysis. Lancet Oncology. 2008;9:222-231.
- Weiss J, Kirsner RS, Hu S. Trends in primary skin cancer prevention among US Hispanics: a systematic review. J Drugs Dermatol. 2012;11:580-586.
- Carvalho LAD, Aguiar FC, Smalley KSM, et al. Acral melanoma: new insights into the immune and genomic landscape. Neoplasia. 2023;46:100947.
- Kolitz E, Lopes F, Arffa M, et al. UV Exposure and the risk of keratinocyte carcinoma in skin of color: a systematic review. JAMA Dermatol. 2022;158:542-546.
- Lukowiak TM, Aizman L, Perz A, et al. Association of age, sex, race, and geographic region with variation of the ratio of basal cell to cutaneous squamous cell carcinomas in the United States. JAMA Dermatol. 2020;156:1192-1198.
- Basset-Seguin N, Herms F. Update in the management of basal cell carcinoma. Acta Derm Venereol. 2020;100:adv00140.
- McDaniel B, Badri T, Steele RB. Basal cell carcinoma. StatPearls [Internet]. Updated March 13, 2024. Accessed September 3, 2024. https://www.ncbi.nlm.nih.gov/books/NBK482439/
- Dessinioti C, Antoniou C, Katsambas A, et al. Basal cell carcinoma: what’s new under the sun. Photochem Photobiol. 2010;86:481-491.
- Kim DP, Kus KJB, Ruiz E. Basal cell carcinoma review. Hematol Oncol Clin North Am. 2019;33:13-24.
- Bigler C, Feldman J, Hall E, et al. Pigmented basal cell carcinoma in Hispanics. J Am Acad Dermatol. 1996;34(5 pt 1):751-752.
- Higgins S, Nazemi A, Chow M, et al. Review of nonmelanoma skin cancer in African Americans, Hispanics, and Asians. Dermatol Surg. 2018;44:903-910.
- Byrd-Miles K, Toombs EL, Peck GL. Skin cancer in individuals of African, Asian, Latin-American, and American-Indian descent: differences in incidence, clinical presentation, and survival compared to Caucasians. J Drugs Dermatol. 2007;6:10-16.
- Rivas M, Rojas E, Calaf GM, et al. Association between non-melanoma and melanoma skin cancer rates, vitamin D and latitude. Oncol Lett. 2017;13:3787-3792.
- Bradford PT. Skin cancer in skin of color. Dermatol Nurs. 2009;21:170-177, 206.
- Davis DS, Robinson C, Callender VD. Skin cancer in women of color: epidemiology, pathogenesis and clinical manifestations. Int J Womens Dermatol. 2021;7:127-134.
- Maafs E, De la Barreda F, Delgado R, et al. Basal cell carcinoma of trunk and extremities. Int J Dermatol. 1997;36:622-628.
- Munjal A, Ferguson N. Skin cancer in skin of color. Dermatol Clin. 2023;41:481-489.
- Jorgenson E, Choquet H, Yin J, et al. Genetic ancestry, skin pigmentation, and the risk of cutaneous squamous cell carcinoma in Hispanic/Latino and non-Hispanic white populations. Commun Biol. 2020;3:765.
- Soliman YS, Mieczkowska K, Zhu TR, et al. Characterizing basal cell carcinoma in Hispanic individuals undergoing Mohs micrographic surgery: a 7-year retrospective review at an academic institution in the Bronx. Brit J Dermatol. 2022;187:597-599.
- Sierro TJ, Blumenthal LY, Hekmatjah J, et al. Differences in health care resource utilization and costs for keratinocyte carcinoma among racioethnic groups: a population-based study. J Am Acad Dermatol. 2022;86:373-378.
- Blumenthal LY, Arzeno J, Syder N, et al. Disparities in nonmelanoma skin cancer in Hispanic/Latino patients based on Mohs micrographic surgery defect size: a multicenter retrospective study. J Am Acad Dermatol. 2022;86:353-358.
- Slopnick EA, Kim SP, Kiechle JE, et al. Racial disparities differ for African Americans and Hispanics in the diagnosis and treatment of penile cancer. Urology. 2016;96:22-28.
- Goodman MT, Hernandez BY, Shvetsov YB. Demographic and pathologic differences in the incidence of invasive penile cancer in the United States, 1995-2003. Cancer Epidemiol Biomarkers Prev. 2007;16:1833-1839.
- Thompson EL, Rosen BL, Maness SB. Social determinants of health and human papillomavirus vaccination among young adults, National Health Interview Survey 2016. J Community Health. 2019;44:149-158.
- Hao X, Billings SD, Wu F, et al. Dermatofibrosarcoma protuberans: update on the diagnosis and treatment. J Clin Med. 2020;9:1752.
- Mosallaei D, Lee EB, Lobl M, et al. Rare cutaneous malignancies in skin of color. Dermatol Surg. 2022;48:606-612.
- Criscito MC, Martires KJ, Stein JA. Prognostic factors, treatment, and survival in dermatofibrosarcoma protuberans. JAMA Dermatol. 2016;152:1365-1371.
- Orenstein LAV, Nelson MM, Wolner Z, et al. Differences in outpatient dermatology encounter work relative value units and net payments by patient race, sex, and age. JAMA Dermatol. 2021;157:406-412.
- Lowe GC, Onajin O, Baum CL, et al. A comparison of Mohs micrographic surgery and wide local excision for treatment of dermatofibrosarcoma protuberans with long-term follow-up: the Mayo Clinic experience. Dermatol Surg. 2017;43:98-106.
- Moore KJ, Chang MS, Weiss J, et al. Racial and ethnic differences in the surgical treatment of dermatofibrosarcoma protuberans: a retrospective cohort analysis. J Am Acad Dermatol. 2022;87:245-247.
- Trofymenko O, Bordeaux JS, Zeitouni NC. Survival in patients with primary dermatofibrosarcoma protuberans: National Cancer Database analysis. J Am Acad Dermatol. 2018;78:1125-1134.
- Bazargan M, Cobb S, Assari S. Discrimination and medical mistrust in a racially and ethnically diverse sample of California adults. Ann Fam Med. 2021;19:4-15.
- Smedley BD, Stith AY, Nelson AR, eds. Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care. Washington, DC; 2003.
- Bailey ZD, Krieger N, Agenor M, et al. Structural racism and health inequities in the USA: evidence and interventions. Lancet. 2017;389:1453-1463.
- Tackett KJ, Jenkins F, Morrell DS, et al. Structural racism and its influence on the severity of atopic dermatitis in African American children. Pediatric Dermatol. 2020;37:142-146.
- Greif C, Srivastava D, Nijhawan RI. A retrospective cohort study of dermatofibrosarcoma protuberans at a large metropolitan academic center. JAAD Int. 2022;6:104-106.
- Office of the Assistant Secretary for Planning and Evaluation. Health insurance coverage and access to care among Latinos: recent rrends and key challenges (HP-2021-22). October 8, 2021. Accessed September 3, 2024. https://aspe.hhs.gov/reports/health-insurance-coverage-access-care-among-latinos
- Keisler-Starkey K, Bunch LN. Health insurance coverage in the United States: 2020 (Current Population Reports No. P60-274). US Census Bureau; 2021. https://www.census.gov/content/dam/Census/library/publications/2021/demo/p60-274.pdf
- Kaiser Family Foundation. Key facts on health coverage of immigrants. Updated June 26, 2024. Accessed September 3, 2024. https://www.kff.org/racial-equity-and-health-policy/fact-sheet/key-facts-on-health-coverage-of-immigrants/
- Pew Research Center. Unauthorized immigrants: length of residency, patterns of parenthood. Published December 1, 2011. Accessed October 28, 2024. https://www.pewresearch.org/race-and-ethnicity/2011/12/01/unauthorized-immigrants-length-of-residency-patterns-of-parenthood/
- Schneider J, Schmitt M. Understanding the relationship between racial discrimination and mental health among African American adults: a review. SAGE Open. 2015;5:1-10.
- Philbin MM, Flake M, Hatzenbuehler ML, et al. State-level immigration and immigrant-focused policies as drivers of Latino health disparities in the United States. Soc Sci Med. 2018;199:29-38.
- Vargas ED, Sanchez GR, Juarez M. The impact of punitive immigrant laws on the health of Latina/o Populations. Polit Policy. 2017;45:312-337.
- Sutton AL, He J, Edmonds MC, et al. Medical mistrust in Black breast cancer patients: acknowledging the roles of the trustor and the trustee. J Cancer Educ. 2019;34:600-607.
- Jacobs J. An overview of Latin American healthcare systems. Pacific Prime Latin America. July 31, 2023. Accessed September 3, 2024. https://www.pacificprime.lat/blog/an-overview-of-latin-american-healthcare-systems/
- CDC. Unfair and unjust practices and conditions harm Hispanic and Latino people and drive health disparities. May 15, 2024. Accessed September 3, 2024. https://www.cdc.gov/tobacco-health-equity/collection/hispanic-latino-unfair-and-unjust.html
- Hall IJ, Rim SH, Dasari S. Preventive care use among Hispanic adults with limited comfort speaking English: an analysis of the Medical Expenditure Panel Survey data. Prev Med. 2022;159:107042.
- Brach C, Chevarley FM. Demographics and health care access and utilization of limited-English-proficient and English-proficient Hispanics. Agency for Healthcare Research and Quality. February 2008. http://meps.ahrq.gov/mepsweb/data_files/publications//rf28/rf28.pdf
- Berdahl TA, Kirby JB. Patient-provider communication disparities by limited English proficiency (LEP): trends from the US Medical Expenditure Panel Survey, 2006-2015. J General Intern Med. 2019;34:1434-1440.
- Robinson JK, Joshi KM, Ortiz S, et al. Melanoma knowledge, perception, and awareness in ethnic minorities in Chicago: recommendations regarding education. Psychooncology. 2011;20:313-320.
- Robinson JK, Nodal M, Chavez L, et al. Enhancing the relevance of skin self-examination for Latinos. JAMA Dermatol. 2017;153:717-718.
- Buchanan Lunsford N, Berktold J, Holman DM, et al. Skin cancer knowledge, awareness, beliefs and preventive behaviors among black and hispanic men and women. Prev Med Rep. 2018;12:203-209.
- Madrigal JM, Correa-Mendez M, Arias JD, et al. Hispanic, Latino/a, Latinx, Latine: disentangling the identities of Hispanic/Latino Americans. National Cancer Institute Division of Cancer Epidemiology & Genetics. October 20, 2022. Accessed September 3, 2024. https://dceg.cancer.gov/about/diversity-inclusion/inclusivity-minute/2022/disentangling-identities-hispanic-latino-americans
- Noe-Bustamnate L, Lopez MH, Krogstad JM. US Hispanic population surpassed 60 million in 2019, but growth has slowed. July 7, 2020. Accessed September 3, 2024. https://www.pewresearch.org/short-reads/2020/07/07/u-s-hispanic-population-surpassed-60-million-in-2019-but-growth-has-slowed/
- Frank C, Lopez MH. Hispanic Americans’ trust in and engagement with science. Pew Research Center. June 14, 2022. Accessed September 3, 2024. https://www.pewresearch.org/wp-content/uploads/sites/20/2022/06/PS_2022.06.14_hispanic-americans-science_REPORT.pdf
- US Census Bureau. Projections of the size and composition of the US population: 2014 to 2060. US Government Printing Office; 2015. Accessed September 5, 2024. https://www.census.gov/content/dam/Census/library/publications/2015/demo/p25-1143.pdf
- Zong J. A mosaic, not a monolith: a profile of the U.S. Latino population, 2000-2020. October 26, 2022. Accessed September 3, 2024. https://latino.ucla.edu/research/latino-population-2000-2020/
- Latinos in California, Texas, New York, Florida and New Jersey. Pew Research Center. March 19, 2004. Accessed September 3, 2024. https://www.pewresearch.org/hispanic/2004/03/19/latinos-in-california-texas-new-york-florida-and-new-jersey/
- Pinheiro PS, Sherman RL, Trapido EJ, et al. Cancer incidence in first generation US Hispanics: Cubans, Mexicans, Puerto Ricans, and new Latinos. Cancer Epidemiol Biomarkers Prev. 2009;18:2162-2169.
- Pinheiro PS, Callahan KE, Kobetz EN. Disaggregated Hispanic groups and cancer: importance, methodology, and current knowledge. In: Ramirez AG, Trapido EJ, eds. Advancing the Science of Cancer in Latinos. Springer; 2020:17-34.
- Qian Y, Johannet P, Sawyers A, et al. The ongoing racial disparities in melanoma: an analysis of the Surveillance, Epidemiology, and End Results database (1975-2016). J Am Acad Dermatol. 2021;84:1585-1593.
- Hogue L, Harvey VM. Basal cell carcinoma, squamous cell carcinoma, and cutaneous melanoma in skin of color patients. Dermatol Clin. 2019;37:519-526.
- Cruzval-O’Reilly E, Lugo-Somolinos A. Melanoma in Hispanics: we may have it all wrong. Cutis. 2020;106:28-30.
- Borrell LN, Elhawary JR, Fuentes-Afflick E, et al. Race and genetic ancestry in medicine—a time for reckoning with racism. N Engl J Med. 2021;384:474-480.
- Lopez MH, Krogstad JM, Passel JS. Who is Hispanic? September 5, 2023. Accessed September 3, 2024. https://www.pewresearch.org/short-reads/2023/09/05/who-is-hispanic/
- Carrasquillo OY, Lambert J, Merritt BG. Comment on “Disparities in nonmelanoma skin cancer in Hispanic/Latino patients based on Mohs micrographic surgery defect size: a multicenter retrospective study.”J Am Acad Dermatol. 2022;87:E129-E130.
- American Cancer Society. Key statistics for melanoma skin cancer. Updated January 17, 2024. Accessed September 3, 2024. https://www.cancer.org/cancer/types/melanoma-skin-cancer/about/key-statistics.html
- National Cancer Institute. Melanoma of the skin: recent trends in SEER age-adjusted incidence rates, 2000-2021. Updated June 27, 2024. Accessed September 3, 2024. https://seer.cancer.gov/statistics-network/explorer/application.htmlsite=53&data_type=1&graph_type=2&compareBy=sex&chk_sex_3=3&chk_sex_2=2&rate_type=2&race=6&age_range=1&stage=101&advopt_precision=1&advopt_show_ci=on&hdn_view=0&advopt_display=2
- Garnett E, Townsend J, Steele B, et al. Characteristics, rates, and trends of melanoma incidence among Hispanics in the USA. Cancer Causes Control. 2016;27:647-659.
- Higgins S, Nazemi A, Feinstein S, et al. Clinical presentations of melanoma in African Americans, Hispanics, and Asians. Dermatol Surg. 2019;45:791-801.
- Bristow IR, de Berker DA, Acland KM, et al. Clinical guidelines for the recognition of melanoma of the foot and nail unit. J Foot Ankle Res. 2010;3:25.
- Fernandez JM, Mata EM, Behbahani S, et al. Survival of Hispanic patients with cutaneous melanoma: a retrospective cohort analysis of 6016 cases from the National Cancer Database. J Am Acad Dermatol. 2023;88:1135-1138.
- Hu S, Sherman R, Arheart K, et al. Predictors of neighborhood risk for late-stage melanoma: addressing disparities through spatial analysis and area-based measures. J Investigative Dermatol. 2014;134:937-945.
- Buster KJ, You Z, Fouad M, et al. Skin cancer risk perceptions: a comparison across ethnicity, age, education, gender, and income. J Am Acad Dermatol. 2012;66:771-779.
- Halpern MT, Ward EM, Pavluck AL, et al. Association of insurance status and ethnicity with cancer stage at diagnosis for 12 cancer sites: a retrospective analysis. Lancet Oncology. 2008;9:222-231.
- Weiss J, Kirsner RS, Hu S. Trends in primary skin cancer prevention among US Hispanics: a systematic review. J Drugs Dermatol. 2012;11:580-586.
- Carvalho LAD, Aguiar FC, Smalley KSM, et al. Acral melanoma: new insights into the immune and genomic landscape. Neoplasia. 2023;46:100947.
- Kolitz E, Lopes F, Arffa M, et al. UV Exposure and the risk of keratinocyte carcinoma in skin of color: a systematic review. JAMA Dermatol. 2022;158:542-546.
- Lukowiak TM, Aizman L, Perz A, et al. Association of age, sex, race, and geographic region with variation of the ratio of basal cell to cutaneous squamous cell carcinomas in the United States. JAMA Dermatol. 2020;156:1192-1198.
- Basset-Seguin N, Herms F. Update in the management of basal cell carcinoma. Acta Derm Venereol. 2020;100:adv00140.
- McDaniel B, Badri T, Steele RB. Basal cell carcinoma. StatPearls [Internet]. Updated March 13, 2024. Accessed September 3, 2024. https://www.ncbi.nlm.nih.gov/books/NBK482439/
- Dessinioti C, Antoniou C, Katsambas A, et al. Basal cell carcinoma: what’s new under the sun. Photochem Photobiol. 2010;86:481-491.
- Kim DP, Kus KJB, Ruiz E. Basal cell carcinoma review. Hematol Oncol Clin North Am. 2019;33:13-24.
- Bigler C, Feldman J, Hall E, et al. Pigmented basal cell carcinoma in Hispanics. J Am Acad Dermatol. 1996;34(5 pt 1):751-752.
- Higgins S, Nazemi A, Chow M, et al. Review of nonmelanoma skin cancer in African Americans, Hispanics, and Asians. Dermatol Surg. 2018;44:903-910.
- Byrd-Miles K, Toombs EL, Peck GL. Skin cancer in individuals of African, Asian, Latin-American, and American-Indian descent: differences in incidence, clinical presentation, and survival compared to Caucasians. J Drugs Dermatol. 2007;6:10-16.
- Rivas M, Rojas E, Calaf GM, et al. Association between non-melanoma and melanoma skin cancer rates, vitamin D and latitude. Oncol Lett. 2017;13:3787-3792.
- Bradford PT. Skin cancer in skin of color. Dermatol Nurs. 2009;21:170-177, 206.
- Davis DS, Robinson C, Callender VD. Skin cancer in women of color: epidemiology, pathogenesis and clinical manifestations. Int J Womens Dermatol. 2021;7:127-134.
- Maafs E, De la Barreda F, Delgado R, et al. Basal cell carcinoma of trunk and extremities. Int J Dermatol. 1997;36:622-628.
- Munjal A, Ferguson N. Skin cancer in skin of color. Dermatol Clin. 2023;41:481-489.
- Jorgenson E, Choquet H, Yin J, et al. Genetic ancestry, skin pigmentation, and the risk of cutaneous squamous cell carcinoma in Hispanic/Latino and non-Hispanic white populations. Commun Biol. 2020;3:765.
- Soliman YS, Mieczkowska K, Zhu TR, et al. Characterizing basal cell carcinoma in Hispanic individuals undergoing Mohs micrographic surgery: a 7-year retrospective review at an academic institution in the Bronx. Brit J Dermatol. 2022;187:597-599.
- Sierro TJ, Blumenthal LY, Hekmatjah J, et al. Differences in health care resource utilization and costs for keratinocyte carcinoma among racioethnic groups: a population-based study. J Am Acad Dermatol. 2022;86:373-378.
- Blumenthal LY, Arzeno J, Syder N, et al. Disparities in nonmelanoma skin cancer in Hispanic/Latino patients based on Mohs micrographic surgery defect size: a multicenter retrospective study. J Am Acad Dermatol. 2022;86:353-358.
- Slopnick EA, Kim SP, Kiechle JE, et al. Racial disparities differ for African Americans and Hispanics in the diagnosis and treatment of penile cancer. Urology. 2016;96:22-28.
- Goodman MT, Hernandez BY, Shvetsov YB. Demographic and pathologic differences in the incidence of invasive penile cancer in the United States, 1995-2003. Cancer Epidemiol Biomarkers Prev. 2007;16:1833-1839.
- Thompson EL, Rosen BL, Maness SB. Social determinants of health and human papillomavirus vaccination among young adults, National Health Interview Survey 2016. J Community Health. 2019;44:149-158.
- Hao X, Billings SD, Wu F, et al. Dermatofibrosarcoma protuberans: update on the diagnosis and treatment. J Clin Med. 2020;9:1752.
- Mosallaei D, Lee EB, Lobl M, et al. Rare cutaneous malignancies in skin of color. Dermatol Surg. 2022;48:606-612.
- Criscito MC, Martires KJ, Stein JA. Prognostic factors, treatment, and survival in dermatofibrosarcoma protuberans. JAMA Dermatol. 2016;152:1365-1371.
- Orenstein LAV, Nelson MM, Wolner Z, et al. Differences in outpatient dermatology encounter work relative value units and net payments by patient race, sex, and age. JAMA Dermatol. 2021;157:406-412.
- Lowe GC, Onajin O, Baum CL, et al. A comparison of Mohs micrographic surgery and wide local excision for treatment of dermatofibrosarcoma protuberans with long-term follow-up: the Mayo Clinic experience. Dermatol Surg. 2017;43:98-106.
- Moore KJ, Chang MS, Weiss J, et al. Racial and ethnic differences in the surgical treatment of dermatofibrosarcoma protuberans: a retrospective cohort analysis. J Am Acad Dermatol. 2022;87:245-247.
- Trofymenko O, Bordeaux JS, Zeitouni NC. Survival in patients with primary dermatofibrosarcoma protuberans: National Cancer Database analysis. J Am Acad Dermatol. 2018;78:1125-1134.
- Bazargan M, Cobb S, Assari S. Discrimination and medical mistrust in a racially and ethnically diverse sample of California adults. Ann Fam Med. 2021;19:4-15.
- Smedley BD, Stith AY, Nelson AR, eds. Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care. Washington, DC; 2003.
- Bailey ZD, Krieger N, Agenor M, et al. Structural racism and health inequities in the USA: evidence and interventions. Lancet. 2017;389:1453-1463.
- Tackett KJ, Jenkins F, Morrell DS, et al. Structural racism and its influence on the severity of atopic dermatitis in African American children. Pediatric Dermatol. 2020;37:142-146.
- Greif C, Srivastava D, Nijhawan RI. A retrospective cohort study of dermatofibrosarcoma protuberans at a large metropolitan academic center. JAAD Int. 2022;6:104-106.
- Office of the Assistant Secretary for Planning and Evaluation. Health insurance coverage and access to care among Latinos: recent rrends and key challenges (HP-2021-22). October 8, 2021. Accessed September 3, 2024. https://aspe.hhs.gov/reports/health-insurance-coverage-access-care-among-latinos
- Keisler-Starkey K, Bunch LN. Health insurance coverage in the United States: 2020 (Current Population Reports No. P60-274). US Census Bureau; 2021. https://www.census.gov/content/dam/Census/library/publications/2021/demo/p60-274.pdf
- Kaiser Family Foundation. Key facts on health coverage of immigrants. Updated June 26, 2024. Accessed September 3, 2024. https://www.kff.org/racial-equity-and-health-policy/fact-sheet/key-facts-on-health-coverage-of-immigrants/
- Pew Research Center. Unauthorized immigrants: length of residency, patterns of parenthood. Published December 1, 2011. Accessed October 28, 2024. https://www.pewresearch.org/race-and-ethnicity/2011/12/01/unauthorized-immigrants-length-of-residency-patterns-of-parenthood/
- Schneider J, Schmitt M. Understanding the relationship between racial discrimination and mental health among African American adults: a review. SAGE Open. 2015;5:1-10.
- Philbin MM, Flake M, Hatzenbuehler ML, et al. State-level immigration and immigrant-focused policies as drivers of Latino health disparities in the United States. Soc Sci Med. 2018;199:29-38.
- Vargas ED, Sanchez GR, Juarez M. The impact of punitive immigrant laws on the health of Latina/o Populations. Polit Policy. 2017;45:312-337.
- Sutton AL, He J, Edmonds MC, et al. Medical mistrust in Black breast cancer patients: acknowledging the roles of the trustor and the trustee. J Cancer Educ. 2019;34:600-607.
- Jacobs J. An overview of Latin American healthcare systems. Pacific Prime Latin America. July 31, 2023. Accessed September 3, 2024. https://www.pacificprime.lat/blog/an-overview-of-latin-american-healthcare-systems/
- CDC. Unfair and unjust practices and conditions harm Hispanic and Latino people and drive health disparities. May 15, 2024. Accessed September 3, 2024. https://www.cdc.gov/tobacco-health-equity/collection/hispanic-latino-unfair-and-unjust.html
- Hall IJ, Rim SH, Dasari S. Preventive care use among Hispanic adults with limited comfort speaking English: an analysis of the Medical Expenditure Panel Survey data. Prev Med. 2022;159:107042.
- Brach C, Chevarley FM. Demographics and health care access and utilization of limited-English-proficient and English-proficient Hispanics. Agency for Healthcare Research and Quality. February 2008. http://meps.ahrq.gov/mepsweb/data_files/publications//rf28/rf28.pdf
- Berdahl TA, Kirby JB. Patient-provider communication disparities by limited English proficiency (LEP): trends from the US Medical Expenditure Panel Survey, 2006-2015. J General Intern Med. 2019;34:1434-1440.
- Robinson JK, Joshi KM, Ortiz S, et al. Melanoma knowledge, perception, and awareness in ethnic minorities in Chicago: recommendations regarding education. Psychooncology. 2011;20:313-320.
- Robinson JK, Nodal M, Chavez L, et al. Enhancing the relevance of skin self-examination for Latinos. JAMA Dermatol. 2017;153:717-718.
- Buchanan Lunsford N, Berktold J, Holman DM, et al. Skin cancer knowledge, awareness, beliefs and preventive behaviors among black and hispanic men and women. Prev Med Rep. 2018;12:203-209.
- Madrigal JM, Correa-Mendez M, Arias JD, et al. Hispanic, Latino/a, Latinx, Latine: disentangling the identities of Hispanic/Latino Americans. National Cancer Institute Division of Cancer Epidemiology & Genetics. October 20, 2022. Accessed September 3, 2024. https://dceg.cancer.gov/about/diversity-inclusion/inclusivity-minute/2022/disentangling-identities-hispanic-latino-americans
Practice Points
- The Latine/Hispanic community—the largest ethnic minoritized group in the United States—is disproportionately affected by disparities in skin cancer outcomes.
- Factors influencing skin cancer outcomes in Latine/Hispanic patients in the United States are complex and multidimensional, including lack of familiarity among dermatologists with skin cancer manifestation in this population compared to non-Hispanic White individuals as well as limited data elucidating risk factors for skin cancer in patients with skin of color and sociocultural factors.
Emerging Insights in Keloid Pathogenesis and Therapeutics
Keloids are fibroproliferative lesions caused by aberrant wound healing in predisposed individuals.1 While keloids have been reported in patients of all races and ethnicities, they most commonly develop in individuals of African or Asian descent.2 Often associated with symptoms such as pain and itching, keloids can be disfiguring and result in poorer quality of life.3 There is a paucity of research on keloid pathogenesis and efficacious therapeutics, particularly in patients with skin of color (SOC). Herein, we outline the current research on keloid treatment and highlight promising new therapies ranging from innovative intralesional techniques to advanced laser-based and biologic therapies.
Deficiencies in Skin of Color Research
Although keloids are 17 times more prevalent in patients with SOC,4 there is a considerable lack of focus on this population in the literature.5 Studies on keloids that include individuals with SOC often group patients of all skin types together, and subgroup analyses are not always performed.6,7 As a result, dermatologists may face considerable challenges in providing effective treatments for keloids in patients with SOC. With few evidence-based options available, patients with SOC who have keloids continue to experience impairments in quality of life.
Common Keloid Therapies
There currently is no gold-standard treatment for keloids. Common therapeutic modalities include intralesional corticosteroids (ILCs), antineoplastic agents and neuromodulators, laser-based devices, and surgical therapies (eg, excision), as well as combined medical and surgical techniques.8
Intralesional Corticosteroids—Minimally invasive ILCs are the first-line treatment in all patients with keloids, regardless of skin phototype. Because keloid formation results from trauma to the skin, ILCs often are recommended to minimize further skin damage.5 One meta-analysis found that ILCs have demonstrated success rates of 50% to 100%9; however, these studies frequently combine ILCs with other treatment modalities, and few studies have focused on the efficacy of ILC monotherapy in patients with SOC.6,10-13
Antineoplastic Agents and Neuromodulators—Certain antineoplastic agents (eg, 5-fluorouracil [5-FU] and bleomycin) and neuromodulators (eg, botulinum toxin A [BTA]) also have been studied in keloid management.8
5-Fluorouracil frequently is combined with ILCs such as triamcinolone (TAC). Combined therapy is more effective than TAC monotherapy in scar height reduction.14,15 Rates of adverse events such as dyspigmentation, atrophy, and telangiectasias also were lower in patients who received combined therapy.14,15 A systematic review found that intralesional bleomycin may be more effective than TAC alone, 5-FU alone, TAC combined with 5-FU, and TAC combined with cryotherapy; however, hyperpigmentation was a common adverse event, occurring in roughly 70% (42/60) of patients.16,17 Additionally, a 2024 meta-analysis evaluated 20 randomized controlled trials comprising 1114 patients treated with intralesional TAC, 5-FU, BTA, verapamil, and/or bleomycin. Botulinum toxin A and TAC plus 5-FU were found to have outstanding therapeutic efficacy for keloids, and rates of adverse events were similar among users of TAC, 5-FU, BTA, and TAC plus 5-FU.18
While antineoplastic agents and BTA may be promising keloid therapies, further studies demonstrating their efficacy and safety profiles are necessary, particularly regarding dyspigmentation as a potential adverse event, as this may be of concern in patients with darker phototypes.
Laser Therapies—Of all treatment modalities, laser-based keloid therapies have been the most robustly studied in SOC. The 2 main types are ablative (eg, CO2, Er:YAG) and nonablative (eg, pulsed dye, Nd:YAG) lasers. Ablative lasers rapidly heat water molecules within the skin, thereby vaporizing the skin cells in a controlled precise process that reduces scar tissue by removing layers of skin. Nonablative lasers target hemoglobin in blood vessels, reducing oxygen supply and inducing collagen remodeling without damaging the epidermis.19
For patients with SOC, lasers carry a risk for postinflammatory hyperpigmentation.20 To address this risk, recent advancements in laser technology and procedural protocols have aimed to minimize the number of passes and utilize cooling devices21; however, many of these recommendations are based on retrospective reviews and small case series. A 2024 meta-analysis comprising 550 patients found that the combination of fractional CO2 laser therapy and 5-FU was the most effective intervention, markedly reducing Vancouver Scar Scale and pliability scores as well as keloid thickness.22 Conversely, pulsed dye lasers were the least effective in terms of improving scar thickness, pigmentation, and pliability when compared to other treatments.
Randomized controlled trials of laser-based therapies in patients with SOC are lacking in the literature. Future studies should focus on calibrating laser-based therapies for those with darker skin tones and examine the efficacy and adverse effects of ablative and nonablative lasers in patients with SOC.
Promising New Keloid Therapies
Keloid disease pathogenesis is incompletely understood, but several new therapeutic targets have been highlighted in the literature, including dupilumab, pentoxifylline, sirtuin 6 (SIRT6) modulators, remdesivir, and needle-assisted electrocoagulation plus pharmacotherapy.
Dupilumab—An anti–IL-4 and IL-13 monoclonal antibody, dupilumab was first approved for the treatment of severe atopic dermatitis. Its use has broadened since its approval, and keloids have been identified as a potential therapeutic target. A 2019 case study described a 53-year-old Black man with severe atopic dermatitis and chronic keloids that regressed with systemic dupilumab therapy.23 This prompted a follow-up case-control study using real-time polymerase chain reaction testing to evaluate Th2 gene expression (IL-4R, IL-13, and CCL18) of lesional and nonlesional tissue in 3 Black patients with chronic keloids and no concurrent atopic dermatitis vs 5 healthy Black controls. Despite the limited sample size, a significant increase in IL-13 and the Th2 chemokine CCL18 was found in patients with keloids compared to controls (P<.05), suggesting that the entire integument of patients with severe keloids is abnormal.23 This finding supports the use of systemic treatments for chronic and multifocal keloid disease. Several subsequent case reports have corroborated the efficacy of systemic and/or intralesional dupilumab.24,25 However, some studies have reported contradictory findings, suggesting the need for high-quality clinical trials.26,27
Pentoxifylline—Pentoxifylline is a methylated xanthine derivative and a nonspecific phosphodiesterase inhibitor used to treat claudication from peripheral artery disease. It also inhibits the proliferation and rate of collagen synthesis of fibroblasts from keloids in vitro.28,29 A 2019 retrospective, open-label pilot study analyzed postsurgical keloid recurrence in 45 patients with 67 unique keloids that were stratified into low- and high-risk groups based on clinical factors including multiple symptomatic keloids, history of recurrence, and family history.30 Both the low- and the high-risk groups were treated with 40 mg/mL intralesional triamcinolone acetonide monthly for 6 months; however, some of the high-risk keloids also received pentoxifylline 300 mg 3 times daily for 6 months. There was a statistically significant decrease in keloid recurrence rate between the high-risk group treated with pentoxifylline and the low-risk group for whom pentoxifylline was not prescribed (P=.015).
Similarly, a randomized clinical trial comparing the efficacy of combination intralesional pentoxifylline and intralesional triamcinolone vs monotherapy with pentoxifylline or triamcinolone found the most significant improvement in the combination cohort with reduction in keloid height (P=.04), pliability (P=.003), and vascularity (P=.05).31 These findings highlight the need for supplementary studies on the use of pentoxifylline for keloid therapy.
SIRT6 Modulators—SIRT6 modulators are an exciting future therapeutic target. In a recent case-control study evaluating the histologic milieu of keloid tissue vs normal skin specimens, the researchers found that selective overexpression of SIRT6 via the use of a recombinant adenovirus in keloid fibroblasts attenuated proliferation, invasion, and collagen synthesis while fostering apoptosis, likely through the suppression of MAPK/ERK pathway activity.32
Remdesivir—The antiviral drug remdesivir has been reported to have pharmacologic activities in a wide range of fibrotic diseases, including keloids. A 2024 study explored the potential effect and mechanisms of remdesivir on skin fibrosis both in vitro and in rodents.33 Remdesivir was found to decrease skin fibrosis and attenuate the gross weight of keloid tissues in vivo, suppress fibroblast activation and autophagy both in vivo and in vitro, dampen fibroblast activation by the TGF-β1/Smad signaling pathway, and inhibit fibroblasts autophagy by the PI3K/Akt/mTOR signaling pathway. These results demonstrate the therapeutic potential of remdesivir for keloid management.
Needle-Assisted Electrocoagulation Plus Pharmacotherapy—A novel needle-assisted electrocoagulation technique combined with pharmacotherapy (corticosteroid and 5-FU injections) was effective in a Chinese clinical trial involving 6 patients with keloids.34 Investigators used Vancouver Scar Scale and both Patient and Observer Scar Assessment Scale scores to grade patients’ scars before treatment and 1 month after the first treatment cycle. They found that ablation combined with pharmacotherapy significantly reduced all 3 scores without any obvious adverse events (P=.004, P=.006, and P=.017, respectively). This novel combination treatment may serve as a safe and effective therapeutic approach for keloid removal.
Final Thoughts
Emerging treatments offer promising new horizons in keloid management; however, the lack of robust, high-quality clinical trials, especially those focusing on
- Téot L, Mustoe TA, Middelkoop E, eds. Textbook on Scar Management: State of the Art Management and Emerging Technologies. Springer; 2020.
- Davis SA, Feldman SR, McMichael AJ. Management of keloids in the United States, 1990-2009: an analysis of the National Ambulatory Medical Care Survey. Dermatol Surg. 2013;39:988-994. doi:10.1111/dsu.12182
- Kassi K, Kouame K, Kouassi A, et al. Quality of life in black African patients with keloid scars. Dermatol Reports. 2020;12:8312. doi:10.4081/dr.2020.8312
- Delaleu J, Charvet E, Petit A. Keloid disease: review with clinical atlas. part I: definitions, history, epidemiology, clinics and diagnosis. Ann Dermatol Venereol. 2023;150:3-15.
doi:10.1016/j.annder.2022.08.010 - Bronte J, Zhou C, Vempati A, et al. A comprehensive review of non-surgical treatments for hypertrophic and keloid scars in skin of color. Clin Cosmet Investig Dermatol.
2024;17:1459-1469. doi:10.2147/CCID.S470997 - Davison SP, Dayan JH, Clemens MW, et al. Efficacy of intralesional 5-fluorouracil and triamcinolone in the treatment of keloids. Aesthet Surg J. 2009;29:40-46. doi:10.1016/j.asj.2008.11.006
- Azzam OA, Bassiouny DA, El-Hawary MS, et al. Treatment of hypertrophic scars and keloids by fractional carbon dioxide laser: a clinical, histological, and immunohistochemical study. Lasers Med Sci. 2016;31:9-18. doi:10.1007/s10103-015-1824-4
- Ekstein SF, Wyles SP, Moran SL, et al. Keloids: a review of therapeutic management. Int J Dermatol. 2021;60:661-671. doi:10.1111/ijd.15159
- Morelli Coppola M, Salzillo R, Segreto F, et al. Triamcinolone acetonide intralesional injection for the treatment of keloid scars: patient selection and perspectives. Clin Cosmet Investig Dermatol. 2018;11:387-396. doi:10.2147/CCID.S133672
- Kant SB, van den Kerckhove E, Colla C, et al. A new treatment of hypertrophic and keloid scars with combined triamcinolone and verapamil: a retrospective study. Eur J Plast Surg. 2018;41:69-80. doi:10.1007/s00238-017-1322-y
- Cohen AJ, Talasila S, Lazarevic B, et al. Combination cryotherapy and intralesional corticosteroid versus steroid monotherapy in the treatment of keloids. J Cosmet Dermatol. 2023;22:932-936. doi:10.1111/jocd.15520
- Tawaranurak N, Pliensiri P, Tawaranurak K. Combination of fractional carbon dioxide laser and topical triamcinolone vs intralesional triamcinolone for keloid treatment: a randomised clinical trial. Int Wound J. 2022;19:1729-1735. doi:10.1111/iwj.13775
- Belie O, Ugburo AO, Mofikoya BO, et al. A comparison of intralesional verapamil and triamcinolone monotherapy in the treatment of keloids in an African population. Niger J Clin Pract. 2021;24:986-992. doi:10.4103/njcp.njcp_474_20
- Khalid FA, Mehrose MY, Saleem M, et al. Comparison of efficacy and safety of intralesional triamcinolone and combination of triamcinolone with 5-fluorouracil in the treatment of keloids and hypertrophic scars: randomised control trial. Burns. 2019;45:69-75. doi:10.1016/j.burns.2018.08.011
- Asilian A, Darougheh A, Shariati F. New combination of triamcinolone, 5-Fluorouracil, and pulsed-dye laser for treatment of keloid and hypertrophic scars. Dermatol Surg. 2006;32:907-915. doi:10.1111/j.1524-4725.2006.32195.x
- Kim WI, Kim S, Cho SW, et al. The efficacy of bleomycin for treating keloid and hypertrophic scar: a systematic review and meta-analysis. J Cosmet Dermatol. 2020;19:3357-3366. doi:10.1111/jocd.13390
- Kabel A, Sabry H, Sorour N, et al. Comparative study between intralesional injection of bleomycin and 5-fluorouracil in the treatment of keloids and hypertrophic scars. J Dermatol Dermatol Surg. 2016;20:32-38.
- Yang HA, Jheng WL, Yu J, et al. Comparative efficacy of drug interventions for keloids: a network meta-analysis. Ann Plast Surg. 2024;92(1S suppl 1
):S52-S59. doi:10.1097/SAP.0000000000003759 - Preissig J, Hamilton K, Markus R. Current laser resurfacing technologies: a review that delves beneath the surface. Semin Plast Surg. 2012;26:109-116. doi:10.1055/s-0032-1329413
- Bin Dakhil A, Shadid A, Altalhab S. Post-inflammatory hyperpigmentation after carbon dioxide laser: review of prevention and risk factors. Dermatol Reports. 2023;15:9703. doi:10.4081/dr.2023.9703
- Kaushik SB, Alexis AF. Nonablative fractional laser resurfacing in skin of color: evidence-based review. J Clin Aesthet Dermatol. 2017;10:51-67.
- Foppiani JA, Khaity A, Al-Dardery NM, et al. Laser therapy in hypertrophic and keloid scars: a systematic review and network meta-analysis. Aesthetic Plast Surg. Published May 17, 2024. doi:10.1007/s00266-024-04027-9
- Diaz A, Tan K, He H, et al. Keloid lesions show increased IL-4/IL-13 signaling and respond to Th2-targeting dupilumab therapy. J Eur Acad Dermatol Venereol. 2020;34:E161-E164. doi:10.1111/jdv.16097
- Min MS,
Mazori DR, Lee MS, et al. Successful treatment of keloids and hypertrophic scars with systemic and intralesional dupilumab. J Drugs Dermatol. 2023;22:1220-1222. doi:10.36849/JDD.6385 - Wittmer A, Finklea L, Joseph J. Effects of dupilumab on keloid stabilization and prevention. JAAD Case Rep. 2023;37:103-105. doi:10.1016/j.jdcr.2023.05.001
- Luk K, Fakhoury J, Ozog D. Nonresponse and progression of diffuse keloids to dupilumab therapy. J Drugs Dermatol. 2022;21:197-199. doi:10.36849/jdd.6252
- Tirgan MH, Uitto J. Lack of efficacy of dupilumab in the treatment of keloid disorder. J Eur Acad Dermatol Venereol. 2022;36:E120-E122. doi:10.1111/jdv.17669
- Berman B, Duncan MR. Pentoxifylline inhibits the proliferation of human fibroblasts derived from keloid, scleroderma and morphoea skin and their production of collagen, glycosaminoglycans and fibronectin. Br J Dermatol. 1990;123:339-346. doi:10.1111/j.1365-
2133.1990.tb06294.x - Berman B, Duncan MR. Pentoxifylline inhibits normal human dermal fibroblast in vitro proliferation, collagen, glycosaminoglycan, and fibronectin production, and increases collagenase activity. J Invest Dermatol. 1989;92:605-610.
- Tan A, Martinez Luna O, Glass DA 2nd. Pentoxifylline for the prevention of postsurgical keloid recurrence. Dermatol Surg. 2020;46:1353-1356. doi:10.1097/DSS.0000000000002090
- Serag-Eldin YMA, Mahmoud WH, Gamea MM, et al. Intralesional pentoxifylline, triamcinolone acetonide, and their combination for treatment of keloid scars. J Cosmet Dermatol. 2021;20:3330-3340. doi:10.1111/jocd.14305
- Zhou T, Chen Y, Wang C, et al. SIRT6 inhibits the proliferation and collagen synthesis of keloid fibroblasts through MAPK/ERK pathway. Discov Med. 2024;36:1430-1440. doi:10.24976/Discov.Med.202436186.133
- Zhang J, Zhang X, Guo X, et al. Remdesivir alleviates skin fibrosis by suppressing TGF-β1 signaling pathway. PLoS One. 2024;19:E0305927.
doi:10.1371/journal.pone.0305927 - Zhao J, Zhai X, Xu Z, et al. Novel needle-type electrocoagulation and combination pharmacotherapy: basic and clinical studies on efficacy and safety in treating keloids. J Cosmet Dermatol. doi:10.1111/jocd.16453
Keloids are fibroproliferative lesions caused by aberrant wound healing in predisposed individuals.1 While keloids have been reported in patients of all races and ethnicities, they most commonly develop in individuals of African or Asian descent.2 Often associated with symptoms such as pain and itching, keloids can be disfiguring and result in poorer quality of life.3 There is a paucity of research on keloid pathogenesis and efficacious therapeutics, particularly in patients with skin of color (SOC). Herein, we outline the current research on keloid treatment and highlight promising new therapies ranging from innovative intralesional techniques to advanced laser-based and biologic therapies.
Deficiencies in Skin of Color Research
Although keloids are 17 times more prevalent in patients with SOC,4 there is a considerable lack of focus on this population in the literature.5 Studies on keloids that include individuals with SOC often group patients of all skin types together, and subgroup analyses are not always performed.6,7 As a result, dermatologists may face considerable challenges in providing effective treatments for keloids in patients with SOC. With few evidence-based options available, patients with SOC who have keloids continue to experience impairments in quality of life.
Common Keloid Therapies
There currently is no gold-standard treatment for keloids. Common therapeutic modalities include intralesional corticosteroids (ILCs), antineoplastic agents and neuromodulators, laser-based devices, and surgical therapies (eg, excision), as well as combined medical and surgical techniques.8
Intralesional Corticosteroids—Minimally invasive ILCs are the first-line treatment in all patients with keloids, regardless of skin phototype. Because keloid formation results from trauma to the skin, ILCs often are recommended to minimize further skin damage.5 One meta-analysis found that ILCs have demonstrated success rates of 50% to 100%9; however, these studies frequently combine ILCs with other treatment modalities, and few studies have focused on the efficacy of ILC monotherapy in patients with SOC.6,10-13
Antineoplastic Agents and Neuromodulators—Certain antineoplastic agents (eg, 5-fluorouracil [5-FU] and bleomycin) and neuromodulators (eg, botulinum toxin A [BTA]) also have been studied in keloid management.8
5-Fluorouracil frequently is combined with ILCs such as triamcinolone (TAC). Combined therapy is more effective than TAC monotherapy in scar height reduction.14,15 Rates of adverse events such as dyspigmentation, atrophy, and telangiectasias also were lower in patients who received combined therapy.14,15 A systematic review found that intralesional bleomycin may be more effective than TAC alone, 5-FU alone, TAC combined with 5-FU, and TAC combined with cryotherapy; however, hyperpigmentation was a common adverse event, occurring in roughly 70% (42/60) of patients.16,17 Additionally, a 2024 meta-analysis evaluated 20 randomized controlled trials comprising 1114 patients treated with intralesional TAC, 5-FU, BTA, verapamil, and/or bleomycin. Botulinum toxin A and TAC plus 5-FU were found to have outstanding therapeutic efficacy for keloids, and rates of adverse events were similar among users of TAC, 5-FU, BTA, and TAC plus 5-FU.18
While antineoplastic agents and BTA may be promising keloid therapies, further studies demonstrating their efficacy and safety profiles are necessary, particularly regarding dyspigmentation as a potential adverse event, as this may be of concern in patients with darker phototypes.
Laser Therapies—Of all treatment modalities, laser-based keloid therapies have been the most robustly studied in SOC. The 2 main types are ablative (eg, CO2, Er:YAG) and nonablative (eg, pulsed dye, Nd:YAG) lasers. Ablative lasers rapidly heat water molecules within the skin, thereby vaporizing the skin cells in a controlled precise process that reduces scar tissue by removing layers of skin. Nonablative lasers target hemoglobin in blood vessels, reducing oxygen supply and inducing collagen remodeling without damaging the epidermis.19
For patients with SOC, lasers carry a risk for postinflammatory hyperpigmentation.20 To address this risk, recent advancements in laser technology and procedural protocols have aimed to minimize the number of passes and utilize cooling devices21; however, many of these recommendations are based on retrospective reviews and small case series. A 2024 meta-analysis comprising 550 patients found that the combination of fractional CO2 laser therapy and 5-FU was the most effective intervention, markedly reducing Vancouver Scar Scale and pliability scores as well as keloid thickness.22 Conversely, pulsed dye lasers were the least effective in terms of improving scar thickness, pigmentation, and pliability when compared to other treatments.
Randomized controlled trials of laser-based therapies in patients with SOC are lacking in the literature. Future studies should focus on calibrating laser-based therapies for those with darker skin tones and examine the efficacy and adverse effects of ablative and nonablative lasers in patients with SOC.
Promising New Keloid Therapies
Keloid disease pathogenesis is incompletely understood, but several new therapeutic targets have been highlighted in the literature, including dupilumab, pentoxifylline, sirtuin 6 (SIRT6) modulators, remdesivir, and needle-assisted electrocoagulation plus pharmacotherapy.
Dupilumab—An anti–IL-4 and IL-13 monoclonal antibody, dupilumab was first approved for the treatment of severe atopic dermatitis. Its use has broadened since its approval, and keloids have been identified as a potential therapeutic target. A 2019 case study described a 53-year-old Black man with severe atopic dermatitis and chronic keloids that regressed with systemic dupilumab therapy.23 This prompted a follow-up case-control study using real-time polymerase chain reaction testing to evaluate Th2 gene expression (IL-4R, IL-13, and CCL18) of lesional and nonlesional tissue in 3 Black patients with chronic keloids and no concurrent atopic dermatitis vs 5 healthy Black controls. Despite the limited sample size, a significant increase in IL-13 and the Th2 chemokine CCL18 was found in patients with keloids compared to controls (P<.05), suggesting that the entire integument of patients with severe keloids is abnormal.23 This finding supports the use of systemic treatments for chronic and multifocal keloid disease. Several subsequent case reports have corroborated the efficacy of systemic and/or intralesional dupilumab.24,25 However, some studies have reported contradictory findings, suggesting the need for high-quality clinical trials.26,27
Pentoxifylline—Pentoxifylline is a methylated xanthine derivative and a nonspecific phosphodiesterase inhibitor used to treat claudication from peripheral artery disease. It also inhibits the proliferation and rate of collagen synthesis of fibroblasts from keloids in vitro.28,29 A 2019 retrospective, open-label pilot study analyzed postsurgical keloid recurrence in 45 patients with 67 unique keloids that were stratified into low- and high-risk groups based on clinical factors including multiple symptomatic keloids, history of recurrence, and family history.30 Both the low- and the high-risk groups were treated with 40 mg/mL intralesional triamcinolone acetonide monthly for 6 months; however, some of the high-risk keloids also received pentoxifylline 300 mg 3 times daily for 6 months. There was a statistically significant decrease in keloid recurrence rate between the high-risk group treated with pentoxifylline and the low-risk group for whom pentoxifylline was not prescribed (P=.015).
Similarly, a randomized clinical trial comparing the efficacy of combination intralesional pentoxifylline and intralesional triamcinolone vs monotherapy with pentoxifylline or triamcinolone found the most significant improvement in the combination cohort with reduction in keloid height (P=.04), pliability (P=.003), and vascularity (P=.05).31 These findings highlight the need for supplementary studies on the use of pentoxifylline for keloid therapy.
SIRT6 Modulators—SIRT6 modulators are an exciting future therapeutic target. In a recent case-control study evaluating the histologic milieu of keloid tissue vs normal skin specimens, the researchers found that selective overexpression of SIRT6 via the use of a recombinant adenovirus in keloid fibroblasts attenuated proliferation, invasion, and collagen synthesis while fostering apoptosis, likely through the suppression of MAPK/ERK pathway activity.32
Remdesivir—The antiviral drug remdesivir has been reported to have pharmacologic activities in a wide range of fibrotic diseases, including keloids. A 2024 study explored the potential effect and mechanisms of remdesivir on skin fibrosis both in vitro and in rodents.33 Remdesivir was found to decrease skin fibrosis and attenuate the gross weight of keloid tissues in vivo, suppress fibroblast activation and autophagy both in vivo and in vitro, dampen fibroblast activation by the TGF-β1/Smad signaling pathway, and inhibit fibroblasts autophagy by the PI3K/Akt/mTOR signaling pathway. These results demonstrate the therapeutic potential of remdesivir for keloid management.
Needle-Assisted Electrocoagulation Plus Pharmacotherapy—A novel needle-assisted electrocoagulation technique combined with pharmacotherapy (corticosteroid and 5-FU injections) was effective in a Chinese clinical trial involving 6 patients with keloids.34 Investigators used Vancouver Scar Scale and both Patient and Observer Scar Assessment Scale scores to grade patients’ scars before treatment and 1 month after the first treatment cycle. They found that ablation combined with pharmacotherapy significantly reduced all 3 scores without any obvious adverse events (P=.004, P=.006, and P=.017, respectively). This novel combination treatment may serve as a safe and effective therapeutic approach for keloid removal.
Final Thoughts
Emerging treatments offer promising new horizons in keloid management; however, the lack of robust, high-quality clinical trials, especially those focusing on
Keloids are fibroproliferative lesions caused by aberrant wound healing in predisposed individuals.1 While keloids have been reported in patients of all races and ethnicities, they most commonly develop in individuals of African or Asian descent.2 Often associated with symptoms such as pain and itching, keloids can be disfiguring and result in poorer quality of life.3 There is a paucity of research on keloid pathogenesis and efficacious therapeutics, particularly in patients with skin of color (SOC). Herein, we outline the current research on keloid treatment and highlight promising new therapies ranging from innovative intralesional techniques to advanced laser-based and biologic therapies.
Deficiencies in Skin of Color Research
Although keloids are 17 times more prevalent in patients with SOC,4 there is a considerable lack of focus on this population in the literature.5 Studies on keloids that include individuals with SOC often group patients of all skin types together, and subgroup analyses are not always performed.6,7 As a result, dermatologists may face considerable challenges in providing effective treatments for keloids in patients with SOC. With few evidence-based options available, patients with SOC who have keloids continue to experience impairments in quality of life.
Common Keloid Therapies
There currently is no gold-standard treatment for keloids. Common therapeutic modalities include intralesional corticosteroids (ILCs), antineoplastic agents and neuromodulators, laser-based devices, and surgical therapies (eg, excision), as well as combined medical and surgical techniques.8
Intralesional Corticosteroids—Minimally invasive ILCs are the first-line treatment in all patients with keloids, regardless of skin phototype. Because keloid formation results from trauma to the skin, ILCs often are recommended to minimize further skin damage.5 One meta-analysis found that ILCs have demonstrated success rates of 50% to 100%9; however, these studies frequently combine ILCs with other treatment modalities, and few studies have focused on the efficacy of ILC monotherapy in patients with SOC.6,10-13
Antineoplastic Agents and Neuromodulators—Certain antineoplastic agents (eg, 5-fluorouracil [5-FU] and bleomycin) and neuromodulators (eg, botulinum toxin A [BTA]) also have been studied in keloid management.8
5-Fluorouracil frequently is combined with ILCs such as triamcinolone (TAC). Combined therapy is more effective than TAC monotherapy in scar height reduction.14,15 Rates of adverse events such as dyspigmentation, atrophy, and telangiectasias also were lower in patients who received combined therapy.14,15 A systematic review found that intralesional bleomycin may be more effective than TAC alone, 5-FU alone, TAC combined with 5-FU, and TAC combined with cryotherapy; however, hyperpigmentation was a common adverse event, occurring in roughly 70% (42/60) of patients.16,17 Additionally, a 2024 meta-analysis evaluated 20 randomized controlled trials comprising 1114 patients treated with intralesional TAC, 5-FU, BTA, verapamil, and/or bleomycin. Botulinum toxin A and TAC plus 5-FU were found to have outstanding therapeutic efficacy for keloids, and rates of adverse events were similar among users of TAC, 5-FU, BTA, and TAC plus 5-FU.18
While antineoplastic agents and BTA may be promising keloid therapies, further studies demonstrating their efficacy and safety profiles are necessary, particularly regarding dyspigmentation as a potential adverse event, as this may be of concern in patients with darker phototypes.
Laser Therapies—Of all treatment modalities, laser-based keloid therapies have been the most robustly studied in SOC. The 2 main types are ablative (eg, CO2, Er:YAG) and nonablative (eg, pulsed dye, Nd:YAG) lasers. Ablative lasers rapidly heat water molecules within the skin, thereby vaporizing the skin cells in a controlled precise process that reduces scar tissue by removing layers of skin. Nonablative lasers target hemoglobin in blood vessels, reducing oxygen supply and inducing collagen remodeling without damaging the epidermis.19
For patients with SOC, lasers carry a risk for postinflammatory hyperpigmentation.20 To address this risk, recent advancements in laser technology and procedural protocols have aimed to minimize the number of passes and utilize cooling devices21; however, many of these recommendations are based on retrospective reviews and small case series. A 2024 meta-analysis comprising 550 patients found that the combination of fractional CO2 laser therapy and 5-FU was the most effective intervention, markedly reducing Vancouver Scar Scale and pliability scores as well as keloid thickness.22 Conversely, pulsed dye lasers were the least effective in terms of improving scar thickness, pigmentation, and pliability when compared to other treatments.
Randomized controlled trials of laser-based therapies in patients with SOC are lacking in the literature. Future studies should focus on calibrating laser-based therapies for those with darker skin tones and examine the efficacy and adverse effects of ablative and nonablative lasers in patients with SOC.
Promising New Keloid Therapies
Keloid disease pathogenesis is incompletely understood, but several new therapeutic targets have been highlighted in the literature, including dupilumab, pentoxifylline, sirtuin 6 (SIRT6) modulators, remdesivir, and needle-assisted electrocoagulation plus pharmacotherapy.
Dupilumab—An anti–IL-4 and IL-13 monoclonal antibody, dupilumab was first approved for the treatment of severe atopic dermatitis. Its use has broadened since its approval, and keloids have been identified as a potential therapeutic target. A 2019 case study described a 53-year-old Black man with severe atopic dermatitis and chronic keloids that regressed with systemic dupilumab therapy.23 This prompted a follow-up case-control study using real-time polymerase chain reaction testing to evaluate Th2 gene expression (IL-4R, IL-13, and CCL18) of lesional and nonlesional tissue in 3 Black patients with chronic keloids and no concurrent atopic dermatitis vs 5 healthy Black controls. Despite the limited sample size, a significant increase in IL-13 and the Th2 chemokine CCL18 was found in patients with keloids compared to controls (P<.05), suggesting that the entire integument of patients with severe keloids is abnormal.23 This finding supports the use of systemic treatments for chronic and multifocal keloid disease. Several subsequent case reports have corroborated the efficacy of systemic and/or intralesional dupilumab.24,25 However, some studies have reported contradictory findings, suggesting the need for high-quality clinical trials.26,27
Pentoxifylline—Pentoxifylline is a methylated xanthine derivative and a nonspecific phosphodiesterase inhibitor used to treat claudication from peripheral artery disease. It also inhibits the proliferation and rate of collagen synthesis of fibroblasts from keloids in vitro.28,29 A 2019 retrospective, open-label pilot study analyzed postsurgical keloid recurrence in 45 patients with 67 unique keloids that were stratified into low- and high-risk groups based on clinical factors including multiple symptomatic keloids, history of recurrence, and family history.30 Both the low- and the high-risk groups were treated with 40 mg/mL intralesional triamcinolone acetonide monthly for 6 months; however, some of the high-risk keloids also received pentoxifylline 300 mg 3 times daily for 6 months. There was a statistically significant decrease in keloid recurrence rate between the high-risk group treated with pentoxifylline and the low-risk group for whom pentoxifylline was not prescribed (P=.015).
Similarly, a randomized clinical trial comparing the efficacy of combination intralesional pentoxifylline and intralesional triamcinolone vs monotherapy with pentoxifylline or triamcinolone found the most significant improvement in the combination cohort with reduction in keloid height (P=.04), pliability (P=.003), and vascularity (P=.05).31 These findings highlight the need for supplementary studies on the use of pentoxifylline for keloid therapy.
SIRT6 Modulators—SIRT6 modulators are an exciting future therapeutic target. In a recent case-control study evaluating the histologic milieu of keloid tissue vs normal skin specimens, the researchers found that selective overexpression of SIRT6 via the use of a recombinant adenovirus in keloid fibroblasts attenuated proliferation, invasion, and collagen synthesis while fostering apoptosis, likely through the suppression of MAPK/ERK pathway activity.32
Remdesivir—The antiviral drug remdesivir has been reported to have pharmacologic activities in a wide range of fibrotic diseases, including keloids. A 2024 study explored the potential effect and mechanisms of remdesivir on skin fibrosis both in vitro and in rodents.33 Remdesivir was found to decrease skin fibrosis and attenuate the gross weight of keloid tissues in vivo, suppress fibroblast activation and autophagy both in vivo and in vitro, dampen fibroblast activation by the TGF-β1/Smad signaling pathway, and inhibit fibroblasts autophagy by the PI3K/Akt/mTOR signaling pathway. These results demonstrate the therapeutic potential of remdesivir for keloid management.
Needle-Assisted Electrocoagulation Plus Pharmacotherapy—A novel needle-assisted electrocoagulation technique combined with pharmacotherapy (corticosteroid and 5-FU injections) was effective in a Chinese clinical trial involving 6 patients with keloids.34 Investigators used Vancouver Scar Scale and both Patient and Observer Scar Assessment Scale scores to grade patients’ scars before treatment and 1 month after the first treatment cycle. They found that ablation combined with pharmacotherapy significantly reduced all 3 scores without any obvious adverse events (P=.004, P=.006, and P=.017, respectively). This novel combination treatment may serve as a safe and effective therapeutic approach for keloid removal.
Final Thoughts
Emerging treatments offer promising new horizons in keloid management; however, the lack of robust, high-quality clinical trials, especially those focusing on
- Téot L, Mustoe TA, Middelkoop E, eds. Textbook on Scar Management: State of the Art Management and Emerging Technologies. Springer; 2020.
- Davis SA, Feldman SR, McMichael AJ. Management of keloids in the United States, 1990-2009: an analysis of the National Ambulatory Medical Care Survey. Dermatol Surg. 2013;39:988-994. doi:10.1111/dsu.12182
- Kassi K, Kouame K, Kouassi A, et al. Quality of life in black African patients with keloid scars. Dermatol Reports. 2020;12:8312. doi:10.4081/dr.2020.8312
- Delaleu J, Charvet E, Petit A. Keloid disease: review with clinical atlas. part I: definitions, history, epidemiology, clinics and diagnosis. Ann Dermatol Venereol. 2023;150:3-15.
doi:10.1016/j.annder.2022.08.010 - Bronte J, Zhou C, Vempati A, et al. A comprehensive review of non-surgical treatments for hypertrophic and keloid scars in skin of color. Clin Cosmet Investig Dermatol.
2024;17:1459-1469. doi:10.2147/CCID.S470997 - Davison SP, Dayan JH, Clemens MW, et al. Efficacy of intralesional 5-fluorouracil and triamcinolone in the treatment of keloids. Aesthet Surg J. 2009;29:40-46. doi:10.1016/j.asj.2008.11.006
- Azzam OA, Bassiouny DA, El-Hawary MS, et al. Treatment of hypertrophic scars and keloids by fractional carbon dioxide laser: a clinical, histological, and immunohistochemical study. Lasers Med Sci. 2016;31:9-18. doi:10.1007/s10103-015-1824-4
- Ekstein SF, Wyles SP, Moran SL, et al. Keloids: a review of therapeutic management. Int J Dermatol. 2021;60:661-671. doi:10.1111/ijd.15159
- Morelli Coppola M, Salzillo R, Segreto F, et al. Triamcinolone acetonide intralesional injection for the treatment of keloid scars: patient selection and perspectives. Clin Cosmet Investig Dermatol. 2018;11:387-396. doi:10.2147/CCID.S133672
- Kant SB, van den Kerckhove E, Colla C, et al. A new treatment of hypertrophic and keloid scars with combined triamcinolone and verapamil: a retrospective study. Eur J Plast Surg. 2018;41:69-80. doi:10.1007/s00238-017-1322-y
- Cohen AJ, Talasila S, Lazarevic B, et al. Combination cryotherapy and intralesional corticosteroid versus steroid monotherapy in the treatment of keloids. J Cosmet Dermatol. 2023;22:932-936. doi:10.1111/jocd.15520
- Tawaranurak N, Pliensiri P, Tawaranurak K. Combination of fractional carbon dioxide laser and topical triamcinolone vs intralesional triamcinolone for keloid treatment: a randomised clinical trial. Int Wound J. 2022;19:1729-1735. doi:10.1111/iwj.13775
- Belie O, Ugburo AO, Mofikoya BO, et al. A comparison of intralesional verapamil and triamcinolone monotherapy in the treatment of keloids in an African population. Niger J Clin Pract. 2021;24:986-992. doi:10.4103/njcp.njcp_474_20
- Khalid FA, Mehrose MY, Saleem M, et al. Comparison of efficacy and safety of intralesional triamcinolone and combination of triamcinolone with 5-fluorouracil in the treatment of keloids and hypertrophic scars: randomised control trial. Burns. 2019;45:69-75. doi:10.1016/j.burns.2018.08.011
- Asilian A, Darougheh A, Shariati F. New combination of triamcinolone, 5-Fluorouracil, and pulsed-dye laser for treatment of keloid and hypertrophic scars. Dermatol Surg. 2006;32:907-915. doi:10.1111/j.1524-4725.2006.32195.x
- Kim WI, Kim S, Cho SW, et al. The efficacy of bleomycin for treating keloid and hypertrophic scar: a systematic review and meta-analysis. J Cosmet Dermatol. 2020;19:3357-3366. doi:10.1111/jocd.13390
- Kabel A, Sabry H, Sorour N, et al. Comparative study between intralesional injection of bleomycin and 5-fluorouracil in the treatment of keloids and hypertrophic scars. J Dermatol Dermatol Surg. 2016;20:32-38.
- Yang HA, Jheng WL, Yu J, et al. Comparative efficacy of drug interventions for keloids: a network meta-analysis. Ann Plast Surg. 2024;92(1S suppl 1
):S52-S59. doi:10.1097/SAP.0000000000003759 - Preissig J, Hamilton K, Markus R. Current laser resurfacing technologies: a review that delves beneath the surface. Semin Plast Surg. 2012;26:109-116. doi:10.1055/s-0032-1329413
- Bin Dakhil A, Shadid A, Altalhab S. Post-inflammatory hyperpigmentation after carbon dioxide laser: review of prevention and risk factors. Dermatol Reports. 2023;15:9703. doi:10.4081/dr.2023.9703
- Kaushik SB, Alexis AF. Nonablative fractional laser resurfacing in skin of color: evidence-based review. J Clin Aesthet Dermatol. 2017;10:51-67.
- Foppiani JA, Khaity A, Al-Dardery NM, et al. Laser therapy in hypertrophic and keloid scars: a systematic review and network meta-analysis. Aesthetic Plast Surg. Published May 17, 2024. doi:10.1007/s00266-024-04027-9
- Diaz A, Tan K, He H, et al. Keloid lesions show increased IL-4/IL-13 signaling and respond to Th2-targeting dupilumab therapy. J Eur Acad Dermatol Venereol. 2020;34:E161-E164. doi:10.1111/jdv.16097
- Min MS,
Mazori DR, Lee MS, et al. Successful treatment of keloids and hypertrophic scars with systemic and intralesional dupilumab. J Drugs Dermatol. 2023;22:1220-1222. doi:10.36849/JDD.6385 - Wittmer A, Finklea L, Joseph J. Effects of dupilumab on keloid stabilization and prevention. JAAD Case Rep. 2023;37:103-105. doi:10.1016/j.jdcr.2023.05.001
- Luk K, Fakhoury J, Ozog D. Nonresponse and progression of diffuse keloids to dupilumab therapy. J Drugs Dermatol. 2022;21:197-199. doi:10.36849/jdd.6252
- Tirgan MH, Uitto J. Lack of efficacy of dupilumab in the treatment of keloid disorder. J Eur Acad Dermatol Venereol. 2022;36:E120-E122. doi:10.1111/jdv.17669
- Berman B, Duncan MR. Pentoxifylline inhibits the proliferation of human fibroblasts derived from keloid, scleroderma and morphoea skin and their production of collagen, glycosaminoglycans and fibronectin. Br J Dermatol. 1990;123:339-346. doi:10.1111/j.1365-
2133.1990.tb06294.x - Berman B, Duncan MR. Pentoxifylline inhibits normal human dermal fibroblast in vitro proliferation, collagen, glycosaminoglycan, and fibronectin production, and increases collagenase activity. J Invest Dermatol. 1989;92:605-610.
- Tan A, Martinez Luna O, Glass DA 2nd. Pentoxifylline for the prevention of postsurgical keloid recurrence. Dermatol Surg. 2020;46:1353-1356. doi:10.1097/DSS.0000000000002090
- Serag-Eldin YMA, Mahmoud WH, Gamea MM, et al. Intralesional pentoxifylline, triamcinolone acetonide, and their combination for treatment of keloid scars. J Cosmet Dermatol. 2021;20:3330-3340. doi:10.1111/jocd.14305
- Zhou T, Chen Y, Wang C, et al. SIRT6 inhibits the proliferation and collagen synthesis of keloid fibroblasts through MAPK/ERK pathway. Discov Med. 2024;36:1430-1440. doi:10.24976/Discov.Med.202436186.133
- Zhang J, Zhang X, Guo X, et al. Remdesivir alleviates skin fibrosis by suppressing TGF-β1 signaling pathway. PLoS One. 2024;19:E0305927.
doi:10.1371/journal.pone.0305927 - Zhao J, Zhai X, Xu Z, et al. Novel needle-type electrocoagulation and combination pharmacotherapy: basic and clinical studies on efficacy and safety in treating keloids. J Cosmet Dermatol. doi:10.1111/jocd.16453
- Téot L, Mustoe TA, Middelkoop E, eds. Textbook on Scar Management: State of the Art Management and Emerging Technologies. Springer; 2020.
- Davis SA, Feldman SR, McMichael AJ. Management of keloids in the United States, 1990-2009: an analysis of the National Ambulatory Medical Care Survey. Dermatol Surg. 2013;39:988-994. doi:10.1111/dsu.12182
- Kassi K, Kouame K, Kouassi A, et al. Quality of life in black African patients with keloid scars. Dermatol Reports. 2020;12:8312. doi:10.4081/dr.2020.8312
- Delaleu J, Charvet E, Petit A. Keloid disease: review with clinical atlas. part I: definitions, history, epidemiology, clinics and diagnosis. Ann Dermatol Venereol. 2023;150:3-15.
doi:10.1016/j.annder.2022.08.010 - Bronte J, Zhou C, Vempati A, et al. A comprehensive review of non-surgical treatments for hypertrophic and keloid scars in skin of color. Clin Cosmet Investig Dermatol.
2024;17:1459-1469. doi:10.2147/CCID.S470997 - Davison SP, Dayan JH, Clemens MW, et al. Efficacy of intralesional 5-fluorouracil and triamcinolone in the treatment of keloids. Aesthet Surg J. 2009;29:40-46. doi:10.1016/j.asj.2008.11.006
- Azzam OA, Bassiouny DA, El-Hawary MS, et al. Treatment of hypertrophic scars and keloids by fractional carbon dioxide laser: a clinical, histological, and immunohistochemical study. Lasers Med Sci. 2016;31:9-18. doi:10.1007/s10103-015-1824-4
- Ekstein SF, Wyles SP, Moran SL, et al. Keloids: a review of therapeutic management. Int J Dermatol. 2021;60:661-671. doi:10.1111/ijd.15159
- Morelli Coppola M, Salzillo R, Segreto F, et al. Triamcinolone acetonide intralesional injection for the treatment of keloid scars: patient selection and perspectives. Clin Cosmet Investig Dermatol. 2018;11:387-396. doi:10.2147/CCID.S133672
- Kant SB, van den Kerckhove E, Colla C, et al. A new treatment of hypertrophic and keloid scars with combined triamcinolone and verapamil: a retrospective study. Eur J Plast Surg. 2018;41:69-80. doi:10.1007/s00238-017-1322-y
- Cohen AJ, Talasila S, Lazarevic B, et al. Combination cryotherapy and intralesional corticosteroid versus steroid monotherapy in the treatment of keloids. J Cosmet Dermatol. 2023;22:932-936. doi:10.1111/jocd.15520
- Tawaranurak N, Pliensiri P, Tawaranurak K. Combination of fractional carbon dioxide laser and topical triamcinolone vs intralesional triamcinolone for keloid treatment: a randomised clinical trial. Int Wound J. 2022;19:1729-1735. doi:10.1111/iwj.13775
- Belie O, Ugburo AO, Mofikoya BO, et al. A comparison of intralesional verapamil and triamcinolone monotherapy in the treatment of keloids in an African population. Niger J Clin Pract. 2021;24:986-992. doi:10.4103/njcp.njcp_474_20
- Khalid FA, Mehrose MY, Saleem M, et al. Comparison of efficacy and safety of intralesional triamcinolone and combination of triamcinolone with 5-fluorouracil in the treatment of keloids and hypertrophic scars: randomised control trial. Burns. 2019;45:69-75. doi:10.1016/j.burns.2018.08.011
- Asilian A, Darougheh A, Shariati F. New combination of triamcinolone, 5-Fluorouracil, and pulsed-dye laser for treatment of keloid and hypertrophic scars. Dermatol Surg. 2006;32:907-915. doi:10.1111/j.1524-4725.2006.32195.x
- Kim WI, Kim S, Cho SW, et al. The efficacy of bleomycin for treating keloid and hypertrophic scar: a systematic review and meta-analysis. J Cosmet Dermatol. 2020;19:3357-3366. doi:10.1111/jocd.13390
- Kabel A, Sabry H, Sorour N, et al. Comparative study between intralesional injection of bleomycin and 5-fluorouracil in the treatment of keloids and hypertrophic scars. J Dermatol Dermatol Surg. 2016;20:32-38.
- Yang HA, Jheng WL, Yu J, et al. Comparative efficacy of drug interventions for keloids: a network meta-analysis. Ann Plast Surg. 2024;92(1S suppl 1
):S52-S59. doi:10.1097/SAP.0000000000003759 - Preissig J, Hamilton K, Markus R. Current laser resurfacing technologies: a review that delves beneath the surface. Semin Plast Surg. 2012;26:109-116. doi:10.1055/s-0032-1329413
- Bin Dakhil A, Shadid A, Altalhab S. Post-inflammatory hyperpigmentation after carbon dioxide laser: review of prevention and risk factors. Dermatol Reports. 2023;15:9703. doi:10.4081/dr.2023.9703
- Kaushik SB, Alexis AF. Nonablative fractional laser resurfacing in skin of color: evidence-based review. J Clin Aesthet Dermatol. 2017;10:51-67.
- Foppiani JA, Khaity A, Al-Dardery NM, et al. Laser therapy in hypertrophic and keloid scars: a systematic review and network meta-analysis. Aesthetic Plast Surg. Published May 17, 2024. doi:10.1007/s00266-024-04027-9
- Diaz A, Tan K, He H, et al. Keloid lesions show increased IL-4/IL-13 signaling and respond to Th2-targeting dupilumab therapy. J Eur Acad Dermatol Venereol. 2020;34:E161-E164. doi:10.1111/jdv.16097
- Min MS,
Mazori DR, Lee MS, et al. Successful treatment of keloids and hypertrophic scars with systemic and intralesional dupilumab. J Drugs Dermatol. 2023;22:1220-1222. doi:10.36849/JDD.6385 - Wittmer A, Finklea L, Joseph J. Effects of dupilumab on keloid stabilization and prevention. JAAD Case Rep. 2023;37:103-105. doi:10.1016/j.jdcr.2023.05.001
- Luk K, Fakhoury J, Ozog D. Nonresponse and progression of diffuse keloids to dupilumab therapy. J Drugs Dermatol. 2022;21:197-199. doi:10.36849/jdd.6252
- Tirgan MH, Uitto J. Lack of efficacy of dupilumab in the treatment of keloid disorder. J Eur Acad Dermatol Venereol. 2022;36:E120-E122. doi:10.1111/jdv.17669
- Berman B, Duncan MR. Pentoxifylline inhibits the proliferation of human fibroblasts derived from keloid, scleroderma and morphoea skin and their production of collagen, glycosaminoglycans and fibronectin. Br J Dermatol. 1990;123:339-346. doi:10.1111/j.1365-
2133.1990.tb06294.x - Berman B, Duncan MR. Pentoxifylline inhibits normal human dermal fibroblast in vitro proliferation, collagen, glycosaminoglycan, and fibronectin production, and increases collagenase activity. J Invest Dermatol. 1989;92:605-610.
- Tan A, Martinez Luna O, Glass DA 2nd. Pentoxifylline for the prevention of postsurgical keloid recurrence. Dermatol Surg. 2020;46:1353-1356. doi:10.1097/DSS.0000000000002090
- Serag-Eldin YMA, Mahmoud WH, Gamea MM, et al. Intralesional pentoxifylline, triamcinolone acetonide, and their combination for treatment of keloid scars. J Cosmet Dermatol. 2021;20:3330-3340. doi:10.1111/jocd.14305
- Zhou T, Chen Y, Wang C, et al. SIRT6 inhibits the proliferation and collagen synthesis of keloid fibroblasts through MAPK/ERK pathway. Discov Med. 2024;36:1430-1440. doi:10.24976/Discov.Med.202436186.133
- Zhang J, Zhang X, Guo X, et al. Remdesivir alleviates skin fibrosis by suppressing TGF-β1 signaling pathway. PLoS One. 2024;19:E0305927.
doi:10.1371/journal.pone.0305927 - Zhao J, Zhai X, Xu Z, et al. Novel needle-type electrocoagulation and combination pharmacotherapy: basic and clinical studies on efficacy and safety in treating keloids. J Cosmet Dermatol. doi:10.1111/jocd.16453
Hyperkeratotic Papules and Black Macules on the Hands
THE DIAGNOSIS: Acral Hemorrhagic Darier Disease
Darier disease (DD), also known as keratosis follicularis, is a rare autosomal-dominant genodermatosis caused by mutations in the ATPase sarcoplasmic/endoplasmic reticulum Ca2+ transporting 2 gene (ATP2A2). This gene encodes the enzyme sarcoplasmic/endoplasmic reticulum calcium ATPase 2, which results in abnormal calcium signaling in keratinocytes and leads to dyskeratosis.1 Darier disease commonly manifests in the second decade of life with hyperkeratotic papules coalescing into plaques, often accompanied by erosions and fissures that cause discomfort and pruritus. Darier disease also is associated with characteristic nail findings such as the classic candy cane nails and V-shaped nicking.
Acral hemorrhagic lesions are a rare manifestation of DD. Clinically, these lesions can manifest as hemorrhagic macules, papules, and/or vesicles, most commonly occurring following local trauma or retinoid use. Patients with these lesions are believed to have either specific mutations in the ATP2A2 gene that impair sarcoplasmic/endoplasmic reticulum calcium ATPase 2 function in the vascular endothelium or a mutation in the sarcoplasmic/endoplasmic reticulum calcium ATPase protein itself, leading to dysregulation of mitochondrial homeostasis from within the cell, provoking oxidative stress and causing detrimental effects on blood vessels.2 Patients with this variant can present with all the features of classic DD concomitantly, with varying symptom severity or distinct clinical features during separate episodic flares, or as the sole manifestation. Other nonclassical lesions of DD include acral keratoderma, giant comedones, keloidlike vegetations, and leucodermic macules (Figure).3
Acral hemorrhagic DD may appear either in isolation or in tandem with more traditional symptoms, necessitating consideration of other possible differential diagnoses such as acrokeratosis verruciformis of Hopf (AKV), porphyria cutanea tarda, bullous lichen planus (BLP), and hemorrhagic lichen sclerosus.
Sometimes regarded as a variant of DD, AKV is an autosomal- dominant genodermatosis characterized by flat or verrucous hyperkeratotic papules on the hands and feet. In AKV, the nails also may be affected, with changes including striations, subungual hyperkeratosis, and V-shaped nicking of the distal nails. Although our patient displayed features of AKV, it has not been associated with acral hemorrhagic macules, making this diagnosis less likely than DD.4
Porphyria cutanea tarda, a condition caused by decreased levels of uroporphyrinogen decarboxylase, also can cause skin manifestations such as blistering as well as increased skin fragility, predominantly in sun-exposed areas.5 Our patient’s lack of photosensitivity and absence of other common symptoms of this disorder, such as hypertrichosis and hyperpigmentation, made porphyria cutanea tarda less likely.
Bullous lichen planus is a rare subtype of lichen planus characterized by tense bullae arising from preexisting lichen planus lesions or appearing de novo, most commonly manifesting on the oral mucosa or the legs.6 The bullae associated with BLP can rupture and form ulcers—a symptom that could potentially be mistaken for hemorrhagic macules like the ones observed in our patient. However, BLP typically is characterized by erythematous, violaceous, polygonal papules commonly appearing on the oral mucosa and the legs with blisters developing near or on pre-existing lichen planus lesions. These are different from the hyperkeratotic papules and leucodermic macules seen in our patient, which aligned more closely with the clinical presentation of DD.
Hemorrhagic lichen sclerosus presents with white atrophic patches and plaques and hemorrhagic bullae, which may resemble the leucodermic macules and hemorrhagic macules of DD. However, hemorrhagic lichen sclerosus most commonly involves the genital area in postmenopausal women. Extragenital manifestations of lichen sclerosus, although less common, can occur and typically manifest on the thighs, buttocks, breasts, back, chest, axillae, shoulders, and wrists.7 Notably, these hemorrhagic lesions typically are surrounded by hypopigmented skin and display an atrophic appearance.
Management of DD can be challenging. General measures include sun protection, heat avoidance, and friction reduction. Retinoids are considered the first-line therapy for severe DD, as they help normalize keratinocyte differentiation and reduce keratotic scaling.8 Topical corticosteroids can help manage inflammation and reduce the risk for secondary infections. Our patient responded well to this treatment approach, with a notable reduction in the number and severity of the hyperkeratotic plaques and resolution of the acral hemorrhagic lesions.
- Savignac M, Edir A, Simon M, et al. Darier disease: a disease model of impaired calcium homeostasis in the skin. Biochim Biophys Acta. 2011;1813:1111-1117. doi:10.1016/j.bbamcr.2010.12.006
- Hong E, Hu R, Posligua A, et al. Acral hemorrhagic Darier disease: a case report of a rare presentation and literature review. JAAD Case Rep. 2023;31:93-96. doi:10.1016/j.jdcr.2022.05.030
- Yeshurun A, Ziv M, Cohen-Barak E, et al. An update on the cutaneous manifestations of Darier disease. J Cutan Med Surg. 2021;25:498-503. doi:10.1177/1203475421999331
- Williams GM, Lincoln M. Acrokeratosis verruciformis of Hopf. In: StatPearls. StatPearls Publishing; May 1, 2023.
- Shah A, Bhatt H. Cutanea tarda porphyria. In: StatPearls. StatPearls Publishing; April 17, 2023.
- Liakopoulou A, Rallis E. Bullous lichen planus—a review. J Dermatol Case Rep. 2017;11:1-4. doi:10.3315/jdcr.2017.1239
- Arnold N, Manway M, Stephenson S, et al. Extragenital bullous lichen sclerosus on the anterior lower extremities: report of a case and literature review. Dermatol Online J. 2017;23:13030
- Haber RN, Dib NG. Management of Darier disease: a review of the literature and update. Indian J Dermatol Venereol Leprol. 2021;87:14-21. doi:10.25259/IJDVL_963_19 /qt8dn3p7kv.
THE DIAGNOSIS: Acral Hemorrhagic Darier Disease
Darier disease (DD), also known as keratosis follicularis, is a rare autosomal-dominant genodermatosis caused by mutations in the ATPase sarcoplasmic/endoplasmic reticulum Ca2+ transporting 2 gene (ATP2A2). This gene encodes the enzyme sarcoplasmic/endoplasmic reticulum calcium ATPase 2, which results in abnormal calcium signaling in keratinocytes and leads to dyskeratosis.1 Darier disease commonly manifests in the second decade of life with hyperkeratotic papules coalescing into plaques, often accompanied by erosions and fissures that cause discomfort and pruritus. Darier disease also is associated with characteristic nail findings such as the classic candy cane nails and V-shaped nicking.
Acral hemorrhagic lesions are a rare manifestation of DD. Clinically, these lesions can manifest as hemorrhagic macules, papules, and/or vesicles, most commonly occurring following local trauma or retinoid use. Patients with these lesions are believed to have either specific mutations in the ATP2A2 gene that impair sarcoplasmic/endoplasmic reticulum calcium ATPase 2 function in the vascular endothelium or a mutation in the sarcoplasmic/endoplasmic reticulum calcium ATPase protein itself, leading to dysregulation of mitochondrial homeostasis from within the cell, provoking oxidative stress and causing detrimental effects on blood vessels.2 Patients with this variant can present with all the features of classic DD concomitantly, with varying symptom severity or distinct clinical features during separate episodic flares, or as the sole manifestation. Other nonclassical lesions of DD include acral keratoderma, giant comedones, keloidlike vegetations, and leucodermic macules (Figure).3

Acral hemorrhagic DD may appear either in isolation or in tandem with more traditional symptoms, necessitating consideration of other possible differential diagnoses such as acrokeratosis verruciformis of Hopf (AKV), porphyria cutanea tarda, bullous lichen planus (BLP), and hemorrhagic lichen sclerosus.
Sometimes regarded as a variant of DD, AKV is an autosomal- dominant genodermatosis characterized by flat or verrucous hyperkeratotic papules on the hands and feet. In AKV, the nails also may be affected, with changes including striations, subungual hyperkeratosis, and V-shaped nicking of the distal nails. Although our patient displayed features of AKV, it has not been associated with acral hemorrhagic macules, making this diagnosis less likely than DD.4
Porphyria cutanea tarda, a condition caused by decreased levels of uroporphyrinogen decarboxylase, also can cause skin manifestations such as blistering as well as increased skin fragility, predominantly in sun-exposed areas.5 Our patient’s lack of photosensitivity and absence of other common symptoms of this disorder, such as hypertrichosis and hyperpigmentation, made porphyria cutanea tarda less likely.
Bullous lichen planus is a rare subtype of lichen planus characterized by tense bullae arising from preexisting lichen planus lesions or appearing de novo, most commonly manifesting on the oral mucosa or the legs.6 The bullae associated with BLP can rupture and form ulcers—a symptom that could potentially be mistaken for hemorrhagic macules like the ones observed in our patient. However, BLP typically is characterized by erythematous, violaceous, polygonal papules commonly appearing on the oral mucosa and the legs with blisters developing near or on pre-existing lichen planus lesions. These are different from the hyperkeratotic papules and leucodermic macules seen in our patient, which aligned more closely with the clinical presentation of DD.
Hemorrhagic lichen sclerosus presents with white atrophic patches and plaques and hemorrhagic bullae, which may resemble the leucodermic macules and hemorrhagic macules of DD. However, hemorrhagic lichen sclerosus most commonly involves the genital area in postmenopausal women. Extragenital manifestations of lichen sclerosus, although less common, can occur and typically manifest on the thighs, buttocks, breasts, back, chest, axillae, shoulders, and wrists.7 Notably, these hemorrhagic lesions typically are surrounded by hypopigmented skin and display an atrophic appearance.
Management of DD can be challenging. General measures include sun protection, heat avoidance, and friction reduction. Retinoids are considered the first-line therapy for severe DD, as they help normalize keratinocyte differentiation and reduce keratotic scaling.8 Topical corticosteroids can help manage inflammation and reduce the risk for secondary infections. Our patient responded well to this treatment approach, with a notable reduction in the number and severity of the hyperkeratotic plaques and resolution of the acral hemorrhagic lesions.
THE DIAGNOSIS: Acral Hemorrhagic Darier Disease
Darier disease (DD), also known as keratosis follicularis, is a rare autosomal-dominant genodermatosis caused by mutations in the ATPase sarcoplasmic/endoplasmic reticulum Ca2+ transporting 2 gene (ATP2A2). This gene encodes the enzyme sarcoplasmic/endoplasmic reticulum calcium ATPase 2, which results in abnormal calcium signaling in keratinocytes and leads to dyskeratosis.1 Darier disease commonly manifests in the second decade of life with hyperkeratotic papules coalescing into plaques, often accompanied by erosions and fissures that cause discomfort and pruritus. Darier disease also is associated with characteristic nail findings such as the classic candy cane nails and V-shaped nicking.
Acral hemorrhagic lesions are a rare manifestation of DD. Clinically, these lesions can manifest as hemorrhagic macules, papules, and/or vesicles, most commonly occurring following local trauma or retinoid use. Patients with these lesions are believed to have either specific mutations in the ATP2A2 gene that impair sarcoplasmic/endoplasmic reticulum calcium ATPase 2 function in the vascular endothelium or a mutation in the sarcoplasmic/endoplasmic reticulum calcium ATPase protein itself, leading to dysregulation of mitochondrial homeostasis from within the cell, provoking oxidative stress and causing detrimental effects on blood vessels.2 Patients with this variant can present with all the features of classic DD concomitantly, with varying symptom severity or distinct clinical features during separate episodic flares, or as the sole manifestation. Other nonclassical lesions of DD include acral keratoderma, giant comedones, keloidlike vegetations, and leucodermic macules (Figure).3

Acral hemorrhagic DD may appear either in isolation or in tandem with more traditional symptoms, necessitating consideration of other possible differential diagnoses such as acrokeratosis verruciformis of Hopf (AKV), porphyria cutanea tarda, bullous lichen planus (BLP), and hemorrhagic lichen sclerosus.
Sometimes regarded as a variant of DD, AKV is an autosomal- dominant genodermatosis characterized by flat or verrucous hyperkeratotic papules on the hands and feet. In AKV, the nails also may be affected, with changes including striations, subungual hyperkeratosis, and V-shaped nicking of the distal nails. Although our patient displayed features of AKV, it has not been associated with acral hemorrhagic macules, making this diagnosis less likely than DD.4
Porphyria cutanea tarda, a condition caused by decreased levels of uroporphyrinogen decarboxylase, also can cause skin manifestations such as blistering as well as increased skin fragility, predominantly in sun-exposed areas.5 Our patient’s lack of photosensitivity and absence of other common symptoms of this disorder, such as hypertrichosis and hyperpigmentation, made porphyria cutanea tarda less likely.
Bullous lichen planus is a rare subtype of lichen planus characterized by tense bullae arising from preexisting lichen planus lesions or appearing de novo, most commonly manifesting on the oral mucosa or the legs.6 The bullae associated with BLP can rupture and form ulcers—a symptom that could potentially be mistaken for hemorrhagic macules like the ones observed in our patient. However, BLP typically is characterized by erythematous, violaceous, polygonal papules commonly appearing on the oral mucosa and the legs with blisters developing near or on pre-existing lichen planus lesions. These are different from the hyperkeratotic papules and leucodermic macules seen in our patient, which aligned more closely with the clinical presentation of DD.
Hemorrhagic lichen sclerosus presents with white atrophic patches and plaques and hemorrhagic bullae, which may resemble the leucodermic macules and hemorrhagic macules of DD. However, hemorrhagic lichen sclerosus most commonly involves the genital area in postmenopausal women. Extragenital manifestations of lichen sclerosus, although less common, can occur and typically manifest on the thighs, buttocks, breasts, back, chest, axillae, shoulders, and wrists.7 Notably, these hemorrhagic lesions typically are surrounded by hypopigmented skin and display an atrophic appearance.
Management of DD can be challenging. General measures include sun protection, heat avoidance, and friction reduction. Retinoids are considered the first-line therapy for severe DD, as they help normalize keratinocyte differentiation and reduce keratotic scaling.8 Topical corticosteroids can help manage inflammation and reduce the risk for secondary infections. Our patient responded well to this treatment approach, with a notable reduction in the number and severity of the hyperkeratotic plaques and resolution of the acral hemorrhagic lesions.
- Savignac M, Edir A, Simon M, et al. Darier disease: a disease model of impaired calcium homeostasis in the skin. Biochim Biophys Acta. 2011;1813:1111-1117. doi:10.1016/j.bbamcr.2010.12.006
- Hong E, Hu R, Posligua A, et al. Acral hemorrhagic Darier disease: a case report of a rare presentation and literature review. JAAD Case Rep. 2023;31:93-96. doi:10.1016/j.jdcr.2022.05.030
- Yeshurun A, Ziv M, Cohen-Barak E, et al. An update on the cutaneous manifestations of Darier disease. J Cutan Med Surg. 2021;25:498-503. doi:10.1177/1203475421999331
- Williams GM, Lincoln M. Acrokeratosis verruciformis of Hopf. In: StatPearls. StatPearls Publishing; May 1, 2023.
- Shah A, Bhatt H. Cutanea tarda porphyria. In: StatPearls. StatPearls Publishing; April 17, 2023.
- Liakopoulou A, Rallis E. Bullous lichen planus—a review. J Dermatol Case Rep. 2017;11:1-4. doi:10.3315/jdcr.2017.1239
- Arnold N, Manway M, Stephenson S, et al. Extragenital bullous lichen sclerosus on the anterior lower extremities: report of a case and literature review. Dermatol Online J. 2017;23:13030
- Haber RN, Dib NG. Management of Darier disease: a review of the literature and update. Indian J Dermatol Venereol Leprol. 2021;87:14-21. doi:10.25259/IJDVL_963_19 /qt8dn3p7kv.
- Savignac M, Edir A, Simon M, et al. Darier disease: a disease model of impaired calcium homeostasis in the skin. Biochim Biophys Acta. 2011;1813:1111-1117. doi:10.1016/j.bbamcr.2010.12.006
- Hong E, Hu R, Posligua A, et al. Acral hemorrhagic Darier disease: a case report of a rare presentation and literature review. JAAD Case Rep. 2023;31:93-96. doi:10.1016/j.jdcr.2022.05.030
- Yeshurun A, Ziv M, Cohen-Barak E, et al. An update on the cutaneous manifestations of Darier disease. J Cutan Med Surg. 2021;25:498-503. doi:10.1177/1203475421999331
- Williams GM, Lincoln M. Acrokeratosis verruciformis of Hopf. In: StatPearls. StatPearls Publishing; May 1, 2023.
- Shah A, Bhatt H. Cutanea tarda porphyria. In: StatPearls. StatPearls Publishing; April 17, 2023.
- Liakopoulou A, Rallis E. Bullous lichen planus—a review. J Dermatol Case Rep. 2017;11:1-4. doi:10.3315/jdcr.2017.1239
- Arnold N, Manway M, Stephenson S, et al. Extragenital bullous lichen sclerosus on the anterior lower extremities: report of a case and literature review. Dermatol Online J. 2017;23:13030
- Haber RN, Dib NG. Management of Darier disease: a review of the literature and update. Indian J Dermatol Venereol Leprol. 2021;87:14-21. doi:10.25259/IJDVL_963_19 /qt8dn3p7kv.
An elderly woman with a long history of hyperkeratotic papules on the abdomen, forearms, dorsal hands, and skinfolds presented with new lesions on the dorsal hands that had developed over the preceding few months after a lapse in treatment with her previous dermatologist. Her medical history was otherwise unremarkable. Physical examination revealed hyperkeratotic papules, black hemorrhagic macules with jagged borders, and a thin hemorrhagic plaque on the dorsal hands. Nail findings were notable for alternating white and red longitudinal bands with nicking of the distal nail plates. She also had scattered leucodermic macules over the trunk, feet, arms, and legs, as well as numerous hyperkeratotic papules coalescing into plaques over the mons pubis and in the inguinal folds.