User login
Pediatric Melanoma Outcomes by Race and Socioeconomic Factors
To the Editor:
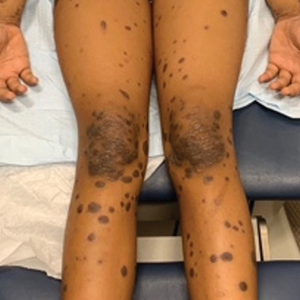
Skin cancers are extremely common worldwide. Malignant melanomas comprise approximately 1 in 5 of these cancers. Exposure to UV radiation is postulated to be responsible for a global rise in melanoma cases over the past 50 years.1 Pediatric melanoma is a particularly rare condition that affects approximately 6 in every 1 million children.2 Melanoma incidence in children ranges by age, increasing by approximately 10-fold from age 1 to 4 years to age 15 to 19 years. Tumor ulceration is a feature more commonly seen among children younger than 10 years and is associated with worse outcomes. Tumor thickness and ulceration strongly predict sentinel lymph node metastases among children, which also is associated with a poor prognosis.3
A recent study evaluating stage IV melanoma survival rates in adolescents and young adults (AYAs) vs older adults found that survival is much worse among AYAs. Thicker tumors and public health insurance also were associated with worse survival rates for AYAs, while early detection was associated with better survival rates.4
Health disparities and their role in the prognosis of pediatric melanoma is another important factor. One study analyzed this relationship at the state level using Texas Cancer Registry data (1995-2009).5 Patients’ socioeconomic status (SES) and driving distance to the nearest pediatric cancer care center were included in the analysis. Hispanic children were found to be 3 times more likely to present with advanced disease than non-Hispanic White children. Although SES and distance to the nearest treatment center were not found to affect the melanoma stage at presentation, Hispanic ethnicity or being in the lowest SES quartile were correlated with a higher mortality risk.5
When considering specific subtypes of melanoma, acral lentiginous melanoma (ALM) is known to develop in patients with skin of color. A 2023 study by Holman et al6 reported that the percentage of melanomas that were ALMs ranged from 0.8% in non-Hispanic White individuals to 19.1% in Hispanic Black, American Indian/Alaska Native, and Asian/Pacific Islander individuals. However, ALM is rare in children. In a pooled cohort study with patient information retrieved from the nationwide Dutch Pathology Registry, only 1 child and 1 adolescent were found to have ALM across a total of 514 patients.7 We sought to analyze pediatric melanoma outcomes based on race and other barriers to appropriate care.
We conducted a search of the Surveillance, Epidemiology, and End Results (SEER) database from January 1995 to December 2016 for patients aged 21 years and younger with a primary melanoma diagnosis. The primary outcome was the 5-year survival rate. County-level SES variables were used to calculate a prosperity index. Kaplan-Meier analysis and Cox proportional hazards model were used to compare 5-year survival rates among the different racial/ethnic groups.
A sample of 2742 patients was identified during the study period and followed for 5 years. Eighty-two percent were White, 6% Hispanic, 2% Asian, 1% Black, and 5% classified as other/unknown race (data were missing for 4%). The cohort was predominantly female (61%). White patients were more likely to present with localized disease than any other race/ethnicity (83% vs 65% in Hispanic, 60% in Asian/Pacific Islander, and 45% in Black patients [P<.05]).
Black and Hispanic patients had the worst 5-year survival rates on bivariate analysis. On multivariate analysis, this finding remained significant for Hispanic patients when compared with White patients (hazard ratio, 2.37 [P<.05]). Increasing age, male sex, advanced stage at diagnosis, and failure to receive surgery were associated with increased odds of mortality.
Patients with regionalized and disseminated disease had increased odds of mortality (6.16 and 64.45, respectively; P<.05) compared with patients with localized disease. Socioeconomic status and urbanization were not found to influence 5-year survival rates.
Pediatric melanoma often presents a clinical challenge with special considerations. Pediatric-specific predisposing risk factors for melanoma and an atypical clinical presentation are some of the major concerns that necessitate a tailored approach to this malignancy, especially among different age groups, skin types, and racial and socioeconomic groups.5
Standard ABCDE criteria often are inadequate for accurate detection of pediatric melanomas. Initial lesions often manifest as raised, red, amelanotic lesions mimicking pyogenic granulomas. Lesions tend to be very small (<6 mm in diameter) and can be uniform in color, thereby making the melanoma more difficult to detect compared to the characteristic findings in adults.5 Bleeding or ulceration often can be a warning sign during physical examination.
With regard to incidence, pediatric melanoma is relatively rare. Since the 1970s, the incidence of pediatric melanoma has been increasing; however, a recent analysis of the SEER database showed a decreasing trend from 2000 to 2010.4
Our analysis of the SEER data showed an increased risk for pediatric melanoma in older adolescents. In addition, the incidence of pediatric melanoma was higher in females of all racial groups except Asian/Pacific Islander individuals. However, SES was not found to significantly influence the 5-year survival rate in pediatric melanoma.
White pediatric patients were more likely to present with localized disease compared with other races. Pediatric melanoma patients with regional disease had a 6-fold increase in mortality rate vs those with localized disease; those with disseminated disease had a 65-fold higher risk. Consistent with this, Black and Hispanic patients had the worst 5-year survival rates on bivariate analysis.
These findings suggest a relationship between race, melanoma spread, and disease severity. Patient education programs need to be directed specifically to minority groups to improve their knowledge on evolving skin lesions and sun protection practices. Physicians also need to have heightened suspicion and better knowledge of the unique traits of pediatric melanoma.5
Given the considerable influence these disparities can have on melanoma outcomes, further research is needed to characterize outcomes based on race and determine obstacles to appropriate care. Improved public outreach initiatives that accommodate specific cultural barriers (eg, language, traditional patterns of behavior) also are required to improve current circumstances.
- Arnold M, Singh D, Laversanne M, et al. Global burden of cutaneous melanoma in 2020 and projections to 2040. JAMA Dermatol. 2022;158:495-503.
- McCormack L, Hawryluk EB. Pediatric melanoma update. G Ital Dermatol Venereol. 2018;153:707-715.
- Saiyed FK, Hamilton EC, Austin MT. Pediatric melanoma: incidence, treatment, and prognosis. Pediatric Health Med Ther. 2017;8:39-45.
- Wojcik KY, Hawkins M, Anderson-Mellies A, et al. Melanoma survival by age group: population-based disparities for adolescent and young adult patients by stage, tumor thickness, and insurance type. J Am Acad Dermatol. 2023;88:831-840.
- Hamilton EC, Nguyen HT, Chang YC, et al. Health disparities influence childhood melanoma stage at diagnosis and outcome. J Pediatr. 2016;175:182-187.
- Holman DM, King JB, White A, et al. Acral lentiginous melanoma incidence by sex, race, ethnicity, and stage in the United States, 2010-2019. Prev Med. 2023;175:107692. doi:10.1016/j.ypmed.2023.107692
- El Sharouni MA, Rawson RV, Potter AJ, et al. Melanomas in children and adolescents: clinicopathologic features and survival outcomes. J Am Acad Dermatol. 2023;88:609-616. doi:10.1016/j.jaad.2022.08.067
To the Editor:
Skin cancers are extremely common worldwide. Malignant melanomas comprise approximately 1 in 5 of these cancers. Exposure to UV radiation is postulated to be responsible for a global rise in melanoma cases over the past 50 years.1 Pediatric melanoma is a particularly rare condition that affects approximately 6 in every 1 million children.2 Melanoma incidence in children ranges by age, increasing by approximately 10-fold from age 1 to 4 years to age 15 to 19 years. Tumor ulceration is a feature more commonly seen among children younger than 10 years and is associated with worse outcomes. Tumor thickness and ulceration strongly predict sentinel lymph node metastases among children, which also is associated with a poor prognosis.3
A recent study evaluating stage IV melanoma survival rates in adolescents and young adults (AYAs) vs older adults found that survival is much worse among AYAs. Thicker tumors and public health insurance also were associated with worse survival rates for AYAs, while early detection was associated with better survival rates.4
Health disparities and their role in the prognosis of pediatric melanoma is another important factor. One study analyzed this relationship at the state level using Texas Cancer Registry data (1995-2009).5 Patients’ socioeconomic status (SES) and driving distance to the nearest pediatric cancer care center were included in the analysis. Hispanic children were found to be 3 times more likely to present with advanced disease than non-Hispanic White children. Although SES and distance to the nearest treatment center were not found to affect the melanoma stage at presentation, Hispanic ethnicity or being in the lowest SES quartile were correlated with a higher mortality risk.5
When considering specific subtypes of melanoma, acral lentiginous melanoma (ALM) is known to develop in patients with skin of color. A 2023 study by Holman et al6 reported that the percentage of melanomas that were ALMs ranged from 0.8% in non-Hispanic White individuals to 19.1% in Hispanic Black, American Indian/Alaska Native, and Asian/Pacific Islander individuals. However, ALM is rare in children. In a pooled cohort study with patient information retrieved from the nationwide Dutch Pathology Registry, only 1 child and 1 adolescent were found to have ALM across a total of 514 patients.7 We sought to analyze pediatric melanoma outcomes based on race and other barriers to appropriate care.
We conducted a search of the Surveillance, Epidemiology, and End Results (SEER) database from January 1995 to December 2016 for patients aged 21 years and younger with a primary melanoma diagnosis. The primary outcome was the 5-year survival rate. County-level SES variables were used to calculate a prosperity index. Kaplan-Meier analysis and Cox proportional hazards model were used to compare 5-year survival rates among the different racial/ethnic groups.
A sample of 2742 patients was identified during the study period and followed for 5 years. Eighty-two percent were White, 6% Hispanic, 2% Asian, 1% Black, and 5% classified as other/unknown race (data were missing for 4%). The cohort was predominantly female (61%). White patients were more likely to present with localized disease than any other race/ethnicity (83% vs 65% in Hispanic, 60% in Asian/Pacific Islander, and 45% in Black patients [P<.05]).
Black and Hispanic patients had the worst 5-year survival rates on bivariate analysis. On multivariate analysis, this finding remained significant for Hispanic patients when compared with White patients (hazard ratio, 2.37 [P<.05]). Increasing age, male sex, advanced stage at diagnosis, and failure to receive surgery were associated with increased odds of mortality.
Patients with regionalized and disseminated disease had increased odds of mortality (6.16 and 64.45, respectively; P<.05) compared with patients with localized disease. Socioeconomic status and urbanization were not found to influence 5-year survival rates.
Pediatric melanoma often presents a clinical challenge with special considerations. Pediatric-specific predisposing risk factors for melanoma and an atypical clinical presentation are some of the major concerns that necessitate a tailored approach to this malignancy, especially among different age groups, skin types, and racial and socioeconomic groups.5
Standard ABCDE criteria often are inadequate for accurate detection of pediatric melanomas. Initial lesions often manifest as raised, red, amelanotic lesions mimicking pyogenic granulomas. Lesions tend to be very small (<6 mm in diameter) and can be uniform in color, thereby making the melanoma more difficult to detect compared to the characteristic findings in adults.5 Bleeding or ulceration often can be a warning sign during physical examination.
With regard to incidence, pediatric melanoma is relatively rare. Since the 1970s, the incidence of pediatric melanoma has been increasing; however, a recent analysis of the SEER database showed a decreasing trend from 2000 to 2010.4
Our analysis of the SEER data showed an increased risk for pediatric melanoma in older adolescents. In addition, the incidence of pediatric melanoma was higher in females of all racial groups except Asian/Pacific Islander individuals. However, SES was not found to significantly influence the 5-year survival rate in pediatric melanoma.
White pediatric patients were more likely to present with localized disease compared with other races. Pediatric melanoma patients with regional disease had a 6-fold increase in mortality rate vs those with localized disease; those with disseminated disease had a 65-fold higher risk. Consistent with this, Black and Hispanic patients had the worst 5-year survival rates on bivariate analysis.
These findings suggest a relationship between race, melanoma spread, and disease severity. Patient education programs need to be directed specifically to minority groups to improve their knowledge on evolving skin lesions and sun protection practices. Physicians also need to have heightened suspicion and better knowledge of the unique traits of pediatric melanoma.5
Given the considerable influence these disparities can have on melanoma outcomes, further research is needed to characterize outcomes based on race and determine obstacles to appropriate care. Improved public outreach initiatives that accommodate specific cultural barriers (eg, language, traditional patterns of behavior) also are required to improve current circumstances.
To the Editor:
Skin cancers are extremely common worldwide. Malignant melanomas comprise approximately 1 in 5 of these cancers. Exposure to UV radiation is postulated to be responsible for a global rise in melanoma cases over the past 50 years.1 Pediatric melanoma is a particularly rare condition that affects approximately 6 in every 1 million children.2 Melanoma incidence in children ranges by age, increasing by approximately 10-fold from age 1 to 4 years to age 15 to 19 years. Tumor ulceration is a feature more commonly seen among children younger than 10 years and is associated with worse outcomes. Tumor thickness and ulceration strongly predict sentinel lymph node metastases among children, which also is associated with a poor prognosis.3
A recent study evaluating stage IV melanoma survival rates in adolescents and young adults (AYAs) vs older adults found that survival is much worse among AYAs. Thicker tumors and public health insurance also were associated with worse survival rates for AYAs, while early detection was associated with better survival rates.4
Health disparities and their role in the prognosis of pediatric melanoma is another important factor. One study analyzed this relationship at the state level using Texas Cancer Registry data (1995-2009).5 Patients’ socioeconomic status (SES) and driving distance to the nearest pediatric cancer care center were included in the analysis. Hispanic children were found to be 3 times more likely to present with advanced disease than non-Hispanic White children. Although SES and distance to the nearest treatment center were not found to affect the melanoma stage at presentation, Hispanic ethnicity or being in the lowest SES quartile were correlated with a higher mortality risk.5
When considering specific subtypes of melanoma, acral lentiginous melanoma (ALM) is known to develop in patients with skin of color. A 2023 study by Holman et al6 reported that the percentage of melanomas that were ALMs ranged from 0.8% in non-Hispanic White individuals to 19.1% in Hispanic Black, American Indian/Alaska Native, and Asian/Pacific Islander individuals. However, ALM is rare in children. In a pooled cohort study with patient information retrieved from the nationwide Dutch Pathology Registry, only 1 child and 1 adolescent were found to have ALM across a total of 514 patients.7 We sought to analyze pediatric melanoma outcomes based on race and other barriers to appropriate care.
We conducted a search of the Surveillance, Epidemiology, and End Results (SEER) database from January 1995 to December 2016 for patients aged 21 years and younger with a primary melanoma diagnosis. The primary outcome was the 5-year survival rate. County-level SES variables were used to calculate a prosperity index. Kaplan-Meier analysis and Cox proportional hazards model were used to compare 5-year survival rates among the different racial/ethnic groups.
A sample of 2742 patients was identified during the study period and followed for 5 years. Eighty-two percent were White, 6% Hispanic, 2% Asian, 1% Black, and 5% classified as other/unknown race (data were missing for 4%). The cohort was predominantly female (61%). White patients were more likely to present with localized disease than any other race/ethnicity (83% vs 65% in Hispanic, 60% in Asian/Pacific Islander, and 45% in Black patients [P<.05]).
Black and Hispanic patients had the worst 5-year survival rates on bivariate analysis. On multivariate analysis, this finding remained significant for Hispanic patients when compared with White patients (hazard ratio, 2.37 [P<.05]). Increasing age, male sex, advanced stage at diagnosis, and failure to receive surgery were associated with increased odds of mortality.
Patients with regionalized and disseminated disease had increased odds of mortality (6.16 and 64.45, respectively; P<.05) compared with patients with localized disease. Socioeconomic status and urbanization were not found to influence 5-year survival rates.
Pediatric melanoma often presents a clinical challenge with special considerations. Pediatric-specific predisposing risk factors for melanoma and an atypical clinical presentation are some of the major concerns that necessitate a tailored approach to this malignancy, especially among different age groups, skin types, and racial and socioeconomic groups.5
Standard ABCDE criteria often are inadequate for accurate detection of pediatric melanomas. Initial lesions often manifest as raised, red, amelanotic lesions mimicking pyogenic granulomas. Lesions tend to be very small (<6 mm in diameter) and can be uniform in color, thereby making the melanoma more difficult to detect compared to the characteristic findings in adults.5 Bleeding or ulceration often can be a warning sign during physical examination.
With regard to incidence, pediatric melanoma is relatively rare. Since the 1970s, the incidence of pediatric melanoma has been increasing; however, a recent analysis of the SEER database showed a decreasing trend from 2000 to 2010.4
Our analysis of the SEER data showed an increased risk for pediatric melanoma in older adolescents. In addition, the incidence of pediatric melanoma was higher in females of all racial groups except Asian/Pacific Islander individuals. However, SES was not found to significantly influence the 5-year survival rate in pediatric melanoma.
White pediatric patients were more likely to present with localized disease compared with other races. Pediatric melanoma patients with regional disease had a 6-fold increase in mortality rate vs those with localized disease; those with disseminated disease had a 65-fold higher risk. Consistent with this, Black and Hispanic patients had the worst 5-year survival rates on bivariate analysis.
These findings suggest a relationship between race, melanoma spread, and disease severity. Patient education programs need to be directed specifically to minority groups to improve their knowledge on evolving skin lesions and sun protection practices. Physicians also need to have heightened suspicion and better knowledge of the unique traits of pediatric melanoma.5
Given the considerable influence these disparities can have on melanoma outcomes, further research is needed to characterize outcomes based on race and determine obstacles to appropriate care. Improved public outreach initiatives that accommodate specific cultural barriers (eg, language, traditional patterns of behavior) also are required to improve current circumstances.
- Arnold M, Singh D, Laversanne M, et al. Global burden of cutaneous melanoma in 2020 and projections to 2040. JAMA Dermatol. 2022;158:495-503.
- McCormack L, Hawryluk EB. Pediatric melanoma update. G Ital Dermatol Venereol. 2018;153:707-715.
- Saiyed FK, Hamilton EC, Austin MT. Pediatric melanoma: incidence, treatment, and prognosis. Pediatric Health Med Ther. 2017;8:39-45.
- Wojcik KY, Hawkins M, Anderson-Mellies A, et al. Melanoma survival by age group: population-based disparities for adolescent and young adult patients by stage, tumor thickness, and insurance type. J Am Acad Dermatol. 2023;88:831-840.
- Hamilton EC, Nguyen HT, Chang YC, et al. Health disparities influence childhood melanoma stage at diagnosis and outcome. J Pediatr. 2016;175:182-187.
- Holman DM, King JB, White A, et al. Acral lentiginous melanoma incidence by sex, race, ethnicity, and stage in the United States, 2010-2019. Prev Med. 2023;175:107692. doi:10.1016/j.ypmed.2023.107692
- El Sharouni MA, Rawson RV, Potter AJ, et al. Melanomas in children and adolescents: clinicopathologic features and survival outcomes. J Am Acad Dermatol. 2023;88:609-616. doi:10.1016/j.jaad.2022.08.067
- Arnold M, Singh D, Laversanne M, et al. Global burden of cutaneous melanoma in 2020 and projections to 2040. JAMA Dermatol. 2022;158:495-503.
- McCormack L, Hawryluk EB. Pediatric melanoma update. G Ital Dermatol Venereol. 2018;153:707-715.
- Saiyed FK, Hamilton EC, Austin MT. Pediatric melanoma: incidence, treatment, and prognosis. Pediatric Health Med Ther. 2017;8:39-45.
- Wojcik KY, Hawkins M, Anderson-Mellies A, et al. Melanoma survival by age group: population-based disparities for adolescent and young adult patients by stage, tumor thickness, and insurance type. J Am Acad Dermatol. 2023;88:831-840.
- Hamilton EC, Nguyen HT, Chang YC, et al. Health disparities influence childhood melanoma stage at diagnosis and outcome. J Pediatr. 2016;175:182-187.
- Holman DM, King JB, White A, et al. Acral lentiginous melanoma incidence by sex, race, ethnicity, and stage in the United States, 2010-2019. Prev Med. 2023;175:107692. doi:10.1016/j.ypmed.2023.107692
- El Sharouni MA, Rawson RV, Potter AJ, et al. Melanomas in children and adolescents: clinicopathologic features and survival outcomes. J Am Acad Dermatol. 2023;88:609-616. doi:10.1016/j.jaad.2022.08.067
Practice Points
- Pediatric melanoma is a unique clinical entity with a different clinical presentation than in adults.
- Thicker tumors and disseminated disease are associated with a worse prognosis, and these factors are more commonly seen in Black and Hispanic patients.
AACR Cancer Progress Report: Big Strides and Big Gaps
The AACR’s 216-page report — an annual endeavor now in its 14th year — focused on the “tremendous” strides made in cancer care, prevention, and early detection and highlighted areas where more research and attention are warranted.
One key area is funding. For the first time since 2016, federal funding for the National Institutes of Health (NIH) and National Cancer Institute (NCI) decreased in the past year. The cuts followed nearly a decade of funding increases that saw the NIH budget expand by nearly $15 billion, and that allowed for a “rapid pace and broad scope” of advances in cancer, AACR’s chief executive officer Margaret Foti, MD, PhD, said during a press briefing.
These recent cuts “threaten to curtail the medical progress seen in recent years and stymie future advancements,” said Dr. Foti, who called on Congress to commit to funding cancer research at significant and consistent levels to “maintain the momentum of progress against cancer.”
Inside the Report: Big Progress
Overall, advances in prevention, early detection, and treatment have helped catch more cancers earlier and save lives.
According to the AACR report, the age-adjusted overall cancer death rate in the United States fell by 33% between 1991 and 2021, meaning about 4.1 million cancer deaths were averted. The overall cancer death rate for children and adolescents has declined by 24% in the past 2 decades. The 5-year relative survival rate for children diagnosed with cancer in the US has improved from 58% for those diagnosed in the mid-1970s to 85% for those diagnosed between 2013 and 2019.
The past fiscal year has seen many new approvals for cancer drugs, diagnostics, and screening tests. From July 1, 2023, to June 30, 2024, the Food and Drug Administration (FDA) approved 15 new anticancer therapeutics, as well as 15 new indications for previously approved agents, one new imaging agent, several artificial intelligence (AI) tools to improve early cancer detection and diagnosis, and two minimally invasive tests for assessing inherited cancer risk or early cancer detection, according to the report.
“Cancer diagnostics are becoming more sophisticated,” AACR president Patricia M. LoRusso, DO, PhD, said during the briefing. “New technologies, such as spatial transcriptomics, are helping us study tumors at a cellular level, and helping to unveil things that we did not initially even begin to understand or think of. AI-based approaches are beginning to transform cancer detection, diagnosis, clinical decision-making, and treatment response monitoring.”
The report also highlights the significant progress in many childhood and adolescent/young adult cancers, Dr. LoRusso noted. These include FDA approvals for two new molecularly targeted therapeutics: tovorafenib for children with certain types of brain tumor and repotrectinib for children with a wide array of cancer types that have a specific genetic alteration known as NTRK gene fusion. It also includes an expanded approval for eflornithine to reduce the risk for relapse in children with high-risk neuroblastoma.
“Decades — decades — of basic research discoveries, have led to these clinical breakthroughs,” she stressed. “These gains against cancer are because of the rapid progress in our ability to decode the cancer genome, which has opened new and innovative avenues for drug development.”
The Gaps
Even with progress in cancer prevention, early detection, and treatment, cancer remains a significant issue.
“In 2024, it is estimated that more than 2 million new cases of cancer will be diagnosed in the United States. More than 611,000 people will die from the disease,” according to the report.
The 2024 report shows that incidence rates for some cancers are increasing in the United States, including vaccine-preventable cancers such as human papillomavirus (HPV)–associated oral cancers and, in young adults, cervical cancers. A recent analysis also found that overall cervical cancer incidence among women aged 30-34 years increased by 2.5% a year between 2012 and 2019.
Furthermore, despite clear evidence demonstrating that the HPV vaccine reduces cervical cancer incidence, uptake has remained poor, with only 38.6% of US children and adolescents aged 9-17 years receiving at least one dose of the vaccine in 2022.
Early-onset cancers are also increasing. Rates of breast, colorectal, and other cancers are on the rise in adults younger than 50 years, the report noted.
The report also pointed to data that 40% of all cancer cases in the United States can be attributed to preventable factors, such as smoking, excess body weight, and alcohol. However, our understanding of these risk factors has improved. Excessive levels of alcohol consumption have, for instance, been shown to increase the risk for six different types of cancer: certain types of head and neck cancer, esophageal squamous cell carcinoma, and breast, colorectal, liver, and stomach cancers.
Financial toxicity remains prevalent as well.
The report explains that financial hardship following a cancer diagnosis is widespread, and the effects can last for years. In fact, more than 40% of patients can spend their entire life savings within the first 2 years of cancer treatment. Among adult survivors of childhood cancers, 20.7% had trouble paying their medical bills, 29.9% said they had been sent to debt collection for unpaid bills, 14.1% had forgone medical care, and 26.8% could not afford nutritious meals.
For young cancer survivors, the lifetime costs associated with a diagnosis of cancer are substantial, reaching an average of $259,324 per person.
On a global level, it is estimated that from 2020 to 2050, the cumulative economic burden of cancer will be $25.2 trillion.
The Path Forward
Despite these challenges, Dr. LoRusso said, “it is unquestionable that we are in a time of unparalleled opportunities in cancer research.
“I am excited about what the future holds for cancer research, and especially for patient care,” she said.
However, funding commitments are needed to avoid impeding this momentum and losing a “talented and creative young workforce” that has brought new ideas and new technologies to the table.
Continued robust funding will help “to markedly improve cancer care, increase cancer survivorship, spur economic growth, and maintain the United States’ position as the global leader in science and medical research,” she added.
The AACR report specifically calls on Congress to:
- Appropriate at least $51.3 billion in fiscal year 2025 for the base budget of the NIH and at least $7.934 billion for the NCI.
- Provide $3.6 billion in dedicated funding for Cancer Moonshot activities through fiscal year 2026 in addition to other funding, consistent with the President’s fiscal year 2025 budget.
- Appropriate at least $472.4 million in fiscal year 2025 for the CDC’s Division of Cancer Prevention to support comprehensive cancer control, central cancer registries, and screening and awareness programs for specific cancers.
- Allocate $55 million in funding for the Oncology Center of Excellence at FDA in fiscal year 2025 to provide regulators with the staff and tools necessary to conduct expedited review of cancer-related medical products.
By working together with Congress and other stakeholders, “we will be able to accelerate the pace of progress and make major strides toward the lifesaving goal of preventing and curing all cancers at the earliest possible time,” Dr. Foti said. “I believe if we do that ... one day we will win this war on cancer.”
A version of this article first appeared on Medscape.com.
The AACR’s 216-page report — an annual endeavor now in its 14th year — focused on the “tremendous” strides made in cancer care, prevention, and early detection and highlighted areas where more research and attention are warranted.
One key area is funding. For the first time since 2016, federal funding for the National Institutes of Health (NIH) and National Cancer Institute (NCI) decreased in the past year. The cuts followed nearly a decade of funding increases that saw the NIH budget expand by nearly $15 billion, and that allowed for a “rapid pace and broad scope” of advances in cancer, AACR’s chief executive officer Margaret Foti, MD, PhD, said during a press briefing.
These recent cuts “threaten to curtail the medical progress seen in recent years and stymie future advancements,” said Dr. Foti, who called on Congress to commit to funding cancer research at significant and consistent levels to “maintain the momentum of progress against cancer.”
Inside the Report: Big Progress
Overall, advances in prevention, early detection, and treatment have helped catch more cancers earlier and save lives.
According to the AACR report, the age-adjusted overall cancer death rate in the United States fell by 33% between 1991 and 2021, meaning about 4.1 million cancer deaths were averted. The overall cancer death rate for children and adolescents has declined by 24% in the past 2 decades. The 5-year relative survival rate for children diagnosed with cancer in the US has improved from 58% for those diagnosed in the mid-1970s to 85% for those diagnosed between 2013 and 2019.
The past fiscal year has seen many new approvals for cancer drugs, diagnostics, and screening tests. From July 1, 2023, to June 30, 2024, the Food and Drug Administration (FDA) approved 15 new anticancer therapeutics, as well as 15 new indications for previously approved agents, one new imaging agent, several artificial intelligence (AI) tools to improve early cancer detection and diagnosis, and two minimally invasive tests for assessing inherited cancer risk or early cancer detection, according to the report.
“Cancer diagnostics are becoming more sophisticated,” AACR president Patricia M. LoRusso, DO, PhD, said during the briefing. “New technologies, such as spatial transcriptomics, are helping us study tumors at a cellular level, and helping to unveil things that we did not initially even begin to understand or think of. AI-based approaches are beginning to transform cancer detection, diagnosis, clinical decision-making, and treatment response monitoring.”
The report also highlights the significant progress in many childhood and adolescent/young adult cancers, Dr. LoRusso noted. These include FDA approvals for two new molecularly targeted therapeutics: tovorafenib for children with certain types of brain tumor and repotrectinib for children with a wide array of cancer types that have a specific genetic alteration known as NTRK gene fusion. It also includes an expanded approval for eflornithine to reduce the risk for relapse in children with high-risk neuroblastoma.
“Decades — decades — of basic research discoveries, have led to these clinical breakthroughs,” she stressed. “These gains against cancer are because of the rapid progress in our ability to decode the cancer genome, which has opened new and innovative avenues for drug development.”
The Gaps
Even with progress in cancer prevention, early detection, and treatment, cancer remains a significant issue.
“In 2024, it is estimated that more than 2 million new cases of cancer will be diagnosed in the United States. More than 611,000 people will die from the disease,” according to the report.
The 2024 report shows that incidence rates for some cancers are increasing in the United States, including vaccine-preventable cancers such as human papillomavirus (HPV)–associated oral cancers and, in young adults, cervical cancers. A recent analysis also found that overall cervical cancer incidence among women aged 30-34 years increased by 2.5% a year between 2012 and 2019.
Furthermore, despite clear evidence demonstrating that the HPV vaccine reduces cervical cancer incidence, uptake has remained poor, with only 38.6% of US children and adolescents aged 9-17 years receiving at least one dose of the vaccine in 2022.
Early-onset cancers are also increasing. Rates of breast, colorectal, and other cancers are on the rise in adults younger than 50 years, the report noted.
The report also pointed to data that 40% of all cancer cases in the United States can be attributed to preventable factors, such as smoking, excess body weight, and alcohol. However, our understanding of these risk factors has improved. Excessive levels of alcohol consumption have, for instance, been shown to increase the risk for six different types of cancer: certain types of head and neck cancer, esophageal squamous cell carcinoma, and breast, colorectal, liver, and stomach cancers.
Financial toxicity remains prevalent as well.
The report explains that financial hardship following a cancer diagnosis is widespread, and the effects can last for years. In fact, more than 40% of patients can spend their entire life savings within the first 2 years of cancer treatment. Among adult survivors of childhood cancers, 20.7% had trouble paying their medical bills, 29.9% said they had been sent to debt collection for unpaid bills, 14.1% had forgone medical care, and 26.8% could not afford nutritious meals.
For young cancer survivors, the lifetime costs associated with a diagnosis of cancer are substantial, reaching an average of $259,324 per person.
On a global level, it is estimated that from 2020 to 2050, the cumulative economic burden of cancer will be $25.2 trillion.
The Path Forward
Despite these challenges, Dr. LoRusso said, “it is unquestionable that we are in a time of unparalleled opportunities in cancer research.
“I am excited about what the future holds for cancer research, and especially for patient care,” she said.
However, funding commitments are needed to avoid impeding this momentum and losing a “talented and creative young workforce” that has brought new ideas and new technologies to the table.
Continued robust funding will help “to markedly improve cancer care, increase cancer survivorship, spur economic growth, and maintain the United States’ position as the global leader in science and medical research,” she added.
The AACR report specifically calls on Congress to:
- Appropriate at least $51.3 billion in fiscal year 2025 for the base budget of the NIH and at least $7.934 billion for the NCI.
- Provide $3.6 billion in dedicated funding for Cancer Moonshot activities through fiscal year 2026 in addition to other funding, consistent with the President’s fiscal year 2025 budget.
- Appropriate at least $472.4 million in fiscal year 2025 for the CDC’s Division of Cancer Prevention to support comprehensive cancer control, central cancer registries, and screening and awareness programs for specific cancers.
- Allocate $55 million in funding for the Oncology Center of Excellence at FDA in fiscal year 2025 to provide regulators with the staff and tools necessary to conduct expedited review of cancer-related medical products.
By working together with Congress and other stakeholders, “we will be able to accelerate the pace of progress and make major strides toward the lifesaving goal of preventing and curing all cancers at the earliest possible time,” Dr. Foti said. “I believe if we do that ... one day we will win this war on cancer.”
A version of this article first appeared on Medscape.com.
The AACR’s 216-page report — an annual endeavor now in its 14th year — focused on the “tremendous” strides made in cancer care, prevention, and early detection and highlighted areas where more research and attention are warranted.
One key area is funding. For the first time since 2016, federal funding for the National Institutes of Health (NIH) and National Cancer Institute (NCI) decreased in the past year. The cuts followed nearly a decade of funding increases that saw the NIH budget expand by nearly $15 billion, and that allowed for a “rapid pace and broad scope” of advances in cancer, AACR’s chief executive officer Margaret Foti, MD, PhD, said during a press briefing.
These recent cuts “threaten to curtail the medical progress seen in recent years and stymie future advancements,” said Dr. Foti, who called on Congress to commit to funding cancer research at significant and consistent levels to “maintain the momentum of progress against cancer.”
Inside the Report: Big Progress
Overall, advances in prevention, early detection, and treatment have helped catch more cancers earlier and save lives.
According to the AACR report, the age-adjusted overall cancer death rate in the United States fell by 33% between 1991 and 2021, meaning about 4.1 million cancer deaths were averted. The overall cancer death rate for children and adolescents has declined by 24% in the past 2 decades. The 5-year relative survival rate for children diagnosed with cancer in the US has improved from 58% for those diagnosed in the mid-1970s to 85% for those diagnosed between 2013 and 2019.
The past fiscal year has seen many new approvals for cancer drugs, diagnostics, and screening tests. From July 1, 2023, to June 30, 2024, the Food and Drug Administration (FDA) approved 15 new anticancer therapeutics, as well as 15 new indications for previously approved agents, one new imaging agent, several artificial intelligence (AI) tools to improve early cancer detection and diagnosis, and two minimally invasive tests for assessing inherited cancer risk or early cancer detection, according to the report.
“Cancer diagnostics are becoming more sophisticated,” AACR president Patricia M. LoRusso, DO, PhD, said during the briefing. “New technologies, such as spatial transcriptomics, are helping us study tumors at a cellular level, and helping to unveil things that we did not initially even begin to understand or think of. AI-based approaches are beginning to transform cancer detection, diagnosis, clinical decision-making, and treatment response monitoring.”
The report also highlights the significant progress in many childhood and adolescent/young adult cancers, Dr. LoRusso noted. These include FDA approvals for two new molecularly targeted therapeutics: tovorafenib for children with certain types of brain tumor and repotrectinib for children with a wide array of cancer types that have a specific genetic alteration known as NTRK gene fusion. It also includes an expanded approval for eflornithine to reduce the risk for relapse in children with high-risk neuroblastoma.
“Decades — decades — of basic research discoveries, have led to these clinical breakthroughs,” she stressed. “These gains against cancer are because of the rapid progress in our ability to decode the cancer genome, which has opened new and innovative avenues for drug development.”
The Gaps
Even with progress in cancer prevention, early detection, and treatment, cancer remains a significant issue.
“In 2024, it is estimated that more than 2 million new cases of cancer will be diagnosed in the United States. More than 611,000 people will die from the disease,” according to the report.
The 2024 report shows that incidence rates for some cancers are increasing in the United States, including vaccine-preventable cancers such as human papillomavirus (HPV)–associated oral cancers and, in young adults, cervical cancers. A recent analysis also found that overall cervical cancer incidence among women aged 30-34 years increased by 2.5% a year between 2012 and 2019.
Furthermore, despite clear evidence demonstrating that the HPV vaccine reduces cervical cancer incidence, uptake has remained poor, with only 38.6% of US children and adolescents aged 9-17 years receiving at least one dose of the vaccine in 2022.
Early-onset cancers are also increasing. Rates of breast, colorectal, and other cancers are on the rise in adults younger than 50 years, the report noted.
The report also pointed to data that 40% of all cancer cases in the United States can be attributed to preventable factors, such as smoking, excess body weight, and alcohol. However, our understanding of these risk factors has improved. Excessive levels of alcohol consumption have, for instance, been shown to increase the risk for six different types of cancer: certain types of head and neck cancer, esophageal squamous cell carcinoma, and breast, colorectal, liver, and stomach cancers.
Financial toxicity remains prevalent as well.
The report explains that financial hardship following a cancer diagnosis is widespread, and the effects can last for years. In fact, more than 40% of patients can spend their entire life savings within the first 2 years of cancer treatment. Among adult survivors of childhood cancers, 20.7% had trouble paying their medical bills, 29.9% said they had been sent to debt collection for unpaid bills, 14.1% had forgone medical care, and 26.8% could not afford nutritious meals.
For young cancer survivors, the lifetime costs associated with a diagnosis of cancer are substantial, reaching an average of $259,324 per person.
On a global level, it is estimated that from 2020 to 2050, the cumulative economic burden of cancer will be $25.2 trillion.
The Path Forward
Despite these challenges, Dr. LoRusso said, “it is unquestionable that we are in a time of unparalleled opportunities in cancer research.
“I am excited about what the future holds for cancer research, and especially for patient care,” she said.
However, funding commitments are needed to avoid impeding this momentum and losing a “talented and creative young workforce” that has brought new ideas and new technologies to the table.
Continued robust funding will help “to markedly improve cancer care, increase cancer survivorship, spur economic growth, and maintain the United States’ position as the global leader in science and medical research,” she added.
The AACR report specifically calls on Congress to:
- Appropriate at least $51.3 billion in fiscal year 2025 for the base budget of the NIH and at least $7.934 billion for the NCI.
- Provide $3.6 billion in dedicated funding for Cancer Moonshot activities through fiscal year 2026 in addition to other funding, consistent with the President’s fiscal year 2025 budget.
- Appropriate at least $472.4 million in fiscal year 2025 for the CDC’s Division of Cancer Prevention to support comprehensive cancer control, central cancer registries, and screening and awareness programs for specific cancers.
- Allocate $55 million in funding for the Oncology Center of Excellence at FDA in fiscal year 2025 to provide regulators with the staff and tools necessary to conduct expedited review of cancer-related medical products.
By working together with Congress and other stakeholders, “we will be able to accelerate the pace of progress and make major strides toward the lifesaving goal of preventing and curing all cancers at the earliest possible time,” Dr. Foti said. “I believe if we do that ... one day we will win this war on cancer.”
A version of this article first appeared on Medscape.com.
The Uneven Surge in Diabetes in the United States
TOPLINE:
METHODOLOGY:
- Over 37 million people in the United States have diabetes, and its prevalence is only expected to increase in the coming years, making identifying high-risk demographic groups particularly crucial.
- To assess recent national trends and disparities in diabetes prevalence among US adults, researchers conducted an observational study using data from the Behavioral Risk Factor Surveillance System and included 5,312,827 observations from 2012 to 2022.
- Diabetes was defined on the basis of a previous self-reported diagnosis using standardized questionnaires.
- The sociodemographic factors of age, sex, race, education, physical activity, income, and body mass index were used to establish the risk indicators for diabetes diagnosis.
- Age-standardized diabetes prevalence and the association between risk factors and diabetes were assessed both overall and across various sociodemographic groups.
TAKEAWAY:
- The overall prevalence of diabetes increased by 18.6% (P < .001) from 2012 to 2022, with the highest prevalence observed among non-Hispanic Black individuals (15.8%) and people aged ≥ 65 years (23.86%).
- The likelihood of being diagnosed with diabetes was 1.15 times higher in men than in women, 5.16 times higher in adults aged 45-64 years than in those aged 18-24 years, and 3.64 times higher in those with obesity than in those with normal weight.
- The risk for being diagnosed with diabetes was 1.60 times higher among Hispanic individuals, 1.67 times higher among non-Hispanic Asian individuals, and 2.10 times higher among non-Hispanic Black individuals than among non-Hispanic White individuals.
- Individuals with a college education and higher income level were 24% and 41% less likely, respectively, to be diagnosed with diabetes.
IN PRACTICE:
“Improving access to quality care, implementing diabetes prevention programs focusing on high-risk groups, and addressing social determinants through multilevel interventions may help curb the diabetes epidemic in the United States,” the authors wrote.
SOURCE:
The study, led by Sulakshan Neupane, MS, Department of Agricultural and Applied Economics, University of Georgia, Athens, Georgia, was published online in Diabetes, Obesity, and Metabolism.
LIMITATIONS:
The self-reported diagnoses and lack of clinical data may have introduced bias. Diabetes prevalence could not be analyzed in South-East Asian and South Asian populations owing to limitations in the data collection process.
DISCLOSURES:
The study was not supported by any funding, and no potential author disclosures or conflicts were identified.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Over 37 million people in the United States have diabetes, and its prevalence is only expected to increase in the coming years, making identifying high-risk demographic groups particularly crucial.
- To assess recent national trends and disparities in diabetes prevalence among US adults, researchers conducted an observational study using data from the Behavioral Risk Factor Surveillance System and included 5,312,827 observations from 2012 to 2022.
- Diabetes was defined on the basis of a previous self-reported diagnosis using standardized questionnaires.
- The sociodemographic factors of age, sex, race, education, physical activity, income, and body mass index were used to establish the risk indicators for diabetes diagnosis.
- Age-standardized diabetes prevalence and the association between risk factors and diabetes were assessed both overall and across various sociodemographic groups.
TAKEAWAY:
- The overall prevalence of diabetes increased by 18.6% (P < .001) from 2012 to 2022, with the highest prevalence observed among non-Hispanic Black individuals (15.8%) and people aged ≥ 65 years (23.86%).
- The likelihood of being diagnosed with diabetes was 1.15 times higher in men than in women, 5.16 times higher in adults aged 45-64 years than in those aged 18-24 years, and 3.64 times higher in those with obesity than in those with normal weight.
- The risk for being diagnosed with diabetes was 1.60 times higher among Hispanic individuals, 1.67 times higher among non-Hispanic Asian individuals, and 2.10 times higher among non-Hispanic Black individuals than among non-Hispanic White individuals.
- Individuals with a college education and higher income level were 24% and 41% less likely, respectively, to be diagnosed with diabetes.
IN PRACTICE:
“Improving access to quality care, implementing diabetes prevention programs focusing on high-risk groups, and addressing social determinants through multilevel interventions may help curb the diabetes epidemic in the United States,” the authors wrote.
SOURCE:
The study, led by Sulakshan Neupane, MS, Department of Agricultural and Applied Economics, University of Georgia, Athens, Georgia, was published online in Diabetes, Obesity, and Metabolism.
LIMITATIONS:
The self-reported diagnoses and lack of clinical data may have introduced bias. Diabetes prevalence could not be analyzed in South-East Asian and South Asian populations owing to limitations in the data collection process.
DISCLOSURES:
The study was not supported by any funding, and no potential author disclosures or conflicts were identified.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Over 37 million people in the United States have diabetes, and its prevalence is only expected to increase in the coming years, making identifying high-risk demographic groups particularly crucial.
- To assess recent national trends and disparities in diabetes prevalence among US adults, researchers conducted an observational study using data from the Behavioral Risk Factor Surveillance System and included 5,312,827 observations from 2012 to 2022.
- Diabetes was defined on the basis of a previous self-reported diagnosis using standardized questionnaires.
- The sociodemographic factors of age, sex, race, education, physical activity, income, and body mass index were used to establish the risk indicators for diabetes diagnosis.
- Age-standardized diabetes prevalence and the association between risk factors and diabetes were assessed both overall and across various sociodemographic groups.
TAKEAWAY:
- The overall prevalence of diabetes increased by 18.6% (P < .001) from 2012 to 2022, with the highest prevalence observed among non-Hispanic Black individuals (15.8%) and people aged ≥ 65 years (23.86%).
- The likelihood of being diagnosed with diabetes was 1.15 times higher in men than in women, 5.16 times higher in adults aged 45-64 years than in those aged 18-24 years, and 3.64 times higher in those with obesity than in those with normal weight.
- The risk for being diagnosed with diabetes was 1.60 times higher among Hispanic individuals, 1.67 times higher among non-Hispanic Asian individuals, and 2.10 times higher among non-Hispanic Black individuals than among non-Hispanic White individuals.
- Individuals with a college education and higher income level were 24% and 41% less likely, respectively, to be diagnosed with diabetes.
IN PRACTICE:
“Improving access to quality care, implementing diabetes prevention programs focusing on high-risk groups, and addressing social determinants through multilevel interventions may help curb the diabetes epidemic in the United States,” the authors wrote.
SOURCE:
The study, led by Sulakshan Neupane, MS, Department of Agricultural and Applied Economics, University of Georgia, Athens, Georgia, was published online in Diabetes, Obesity, and Metabolism.
LIMITATIONS:
The self-reported diagnoses and lack of clinical data may have introduced bias. Diabetes prevalence could not be analyzed in South-East Asian and South Asian populations owing to limitations in the data collection process.
DISCLOSURES:
The study was not supported by any funding, and no potential author disclosures or conflicts were identified.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Black Women Have a Higher Risk for Death in BC Subtypes
TOPLINE:
The greatest disparity in BC-specific survival was observed in those with hormone receptor-positive (HR+), human epidermal growth factor 2–negative (HER2−) tumors, with Black women having a 50% higher risk for death.
METHODOLOGY:
- US Black women have a 40% higher risk for death from BC than White women, and many cancer specialists believe that disparities are worse among more treatable subtypes, such as HR+ tumors.
- Researchers conducted a systematic review and meta-analysis of 18 US studies published during 2009-2022 that included 228,885 women (34,262 Black women; 182,466 White women) and examined racial differences in BC survival by subtype.
- The analysis included hormone receptor and HER2/neu status to define subtypes: HR+ HER2+, HR+ HER2−, HR− HER2+, and HR− HER2−.
- Random-effects models were used to generate pooled relative risks and 95% CI for BC-specific survival and overall survival.
- The primary outcome was BC-specific survival, with overall survival as a secondary analysis.
TAKEAWAY:
- Black women had a higher risk for BC death across all tumor subtypes than White women, with the greatest disparity observed in HR+ HER2− tumors (hazard ratio [HR], 1.50; 95% CI, 1.30-1.72).
- The risk for BC death was also higher for Black women with HR+ HER2+ tumors (HR, 1.34; 95% CI, 1.10-1.64); HR− HER2+ tumors (HR, 1.20; 95% CI, 1.00-1.43); and HR− HER2− tumors (HR, 1.17; 95% CI, 1.10-1.25).
- Overall survival was poorer for Black women across all subtypes, although estimates for HR− HER2+ tumors did not reach statistical significance.
- In analysis of two subtypes with significant heterogeneity among studies, adjustments for socioeconomic status and number of Black participants explained about half and all the variance for HR+ HER2− and HR− HER2+ tumors, respectively.
IN PRACTICE:
“These results suggest there are both subtype-specific and subtype-independent mechanisms that contribute to disparities in breast cancer survival between Black and White women, which require multilevel interventions to address and achieve health equity,” wrote the authors.
SOURCE:
The study was led by Juliana M. Torres, Dana-Farber/Harvard Cancer Center, CURE Program, Boston. It was published online in the Journal of Clinical Oncology.
LIMITATIONS:
The study’s limitations included potential heterogeneity between studies as indicated by significant heterogeneity in some analyses. The use of different subtype definitions and potential overlap in data sets may have also affected the results. Many included studies did not capture the extent to which treatments were completed or detection and treatment of recurrences. Additionally, the study’s findings may not fully capture socioeconomic inequality and other unmeasured factors contributing to disparities. The racial and ethnic disparities analysis focused only on Black and White women.
DISCLOSURES:
Individual authors disclosed financial relationships with Pfizer, Healthix, Merck, AstraZeneca, LabCorp, and Takeda. Additional disclosures are noted in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article appeared on Medscape.com.
TOPLINE:
The greatest disparity in BC-specific survival was observed in those with hormone receptor-positive (HR+), human epidermal growth factor 2–negative (HER2−) tumors, with Black women having a 50% higher risk for death.
METHODOLOGY:
- US Black women have a 40% higher risk for death from BC than White women, and many cancer specialists believe that disparities are worse among more treatable subtypes, such as HR+ tumors.
- Researchers conducted a systematic review and meta-analysis of 18 US studies published during 2009-2022 that included 228,885 women (34,262 Black women; 182,466 White women) and examined racial differences in BC survival by subtype.
- The analysis included hormone receptor and HER2/neu status to define subtypes: HR+ HER2+, HR+ HER2−, HR− HER2+, and HR− HER2−.
- Random-effects models were used to generate pooled relative risks and 95% CI for BC-specific survival and overall survival.
- The primary outcome was BC-specific survival, with overall survival as a secondary analysis.
TAKEAWAY:
- Black women had a higher risk for BC death across all tumor subtypes than White women, with the greatest disparity observed in HR+ HER2− tumors (hazard ratio [HR], 1.50; 95% CI, 1.30-1.72).
- The risk for BC death was also higher for Black women with HR+ HER2+ tumors (HR, 1.34; 95% CI, 1.10-1.64); HR− HER2+ tumors (HR, 1.20; 95% CI, 1.00-1.43); and HR− HER2− tumors (HR, 1.17; 95% CI, 1.10-1.25).
- Overall survival was poorer for Black women across all subtypes, although estimates for HR− HER2+ tumors did not reach statistical significance.
- In analysis of two subtypes with significant heterogeneity among studies, adjustments for socioeconomic status and number of Black participants explained about half and all the variance for HR+ HER2− and HR− HER2+ tumors, respectively.
IN PRACTICE:
“These results suggest there are both subtype-specific and subtype-independent mechanisms that contribute to disparities in breast cancer survival between Black and White women, which require multilevel interventions to address and achieve health equity,” wrote the authors.
SOURCE:
The study was led by Juliana M. Torres, Dana-Farber/Harvard Cancer Center, CURE Program, Boston. It was published online in the Journal of Clinical Oncology.
LIMITATIONS:
The study’s limitations included potential heterogeneity between studies as indicated by significant heterogeneity in some analyses. The use of different subtype definitions and potential overlap in data sets may have also affected the results. Many included studies did not capture the extent to which treatments were completed or detection and treatment of recurrences. Additionally, the study’s findings may not fully capture socioeconomic inequality and other unmeasured factors contributing to disparities. The racial and ethnic disparities analysis focused only on Black and White women.
DISCLOSURES:
Individual authors disclosed financial relationships with Pfizer, Healthix, Merck, AstraZeneca, LabCorp, and Takeda. Additional disclosures are noted in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article appeared on Medscape.com.
TOPLINE:
The greatest disparity in BC-specific survival was observed in those with hormone receptor-positive (HR+), human epidermal growth factor 2–negative (HER2−) tumors, with Black women having a 50% higher risk for death.
METHODOLOGY:
- US Black women have a 40% higher risk for death from BC than White women, and many cancer specialists believe that disparities are worse among more treatable subtypes, such as HR+ tumors.
- Researchers conducted a systematic review and meta-analysis of 18 US studies published during 2009-2022 that included 228,885 women (34,262 Black women; 182,466 White women) and examined racial differences in BC survival by subtype.
- The analysis included hormone receptor and HER2/neu status to define subtypes: HR+ HER2+, HR+ HER2−, HR− HER2+, and HR− HER2−.
- Random-effects models were used to generate pooled relative risks and 95% CI for BC-specific survival and overall survival.
- The primary outcome was BC-specific survival, with overall survival as a secondary analysis.
TAKEAWAY:
- Black women had a higher risk for BC death across all tumor subtypes than White women, with the greatest disparity observed in HR+ HER2− tumors (hazard ratio [HR], 1.50; 95% CI, 1.30-1.72).
- The risk for BC death was also higher for Black women with HR+ HER2+ tumors (HR, 1.34; 95% CI, 1.10-1.64); HR− HER2+ tumors (HR, 1.20; 95% CI, 1.00-1.43); and HR− HER2− tumors (HR, 1.17; 95% CI, 1.10-1.25).
- Overall survival was poorer for Black women across all subtypes, although estimates for HR− HER2+ tumors did not reach statistical significance.
- In analysis of two subtypes with significant heterogeneity among studies, adjustments for socioeconomic status and number of Black participants explained about half and all the variance for HR+ HER2− and HR− HER2+ tumors, respectively.
IN PRACTICE:
“These results suggest there are both subtype-specific and subtype-independent mechanisms that contribute to disparities in breast cancer survival between Black and White women, which require multilevel interventions to address and achieve health equity,” wrote the authors.
SOURCE:
The study was led by Juliana M. Torres, Dana-Farber/Harvard Cancer Center, CURE Program, Boston. It was published online in the Journal of Clinical Oncology.
LIMITATIONS:
The study’s limitations included potential heterogeneity between studies as indicated by significant heterogeneity in some analyses. The use of different subtype definitions and potential overlap in data sets may have also affected the results. Many included studies did not capture the extent to which treatments were completed or detection and treatment of recurrences. Additionally, the study’s findings may not fully capture socioeconomic inequality and other unmeasured factors contributing to disparities. The racial and ethnic disparities analysis focused only on Black and White women.
DISCLOSURES:
Individual authors disclosed financial relationships with Pfizer, Healthix, Merck, AstraZeneca, LabCorp, and Takeda. Additional disclosures are noted in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article appeared on Medscape.com.
Seborrheic Dermatitis in Black Patients: New Therapies Offer Hope
NEW YORK — not only in this group but also overall, now that there is an approved therapy with an array of alternatives and adjunctive medications, according to Shawn Kwatra, MD.
The list of therapies effective against SD, often employed in combination, is lengthy, but topical 0.3% roflumilast foam (Zoryve), approved by the Food and Drug Administration (FDA) late last year for treating SD, has a high rate of efficacy and should now be considered a first-line treatment option, according to Dr. Kwatra, professor and chair of the Department of Dermatology, University of Maryland School of Medicine, Baltimore.
New Approved Therapy Draws Attention to SD
Emphasizing that topical roflumilast does not necessarily replace the use of over-the-counter shampoos and emollients or a list of prescription drugs used off-label to control this condition, he said it is also important for another reason.
“It shines a light on this disease,” said Dr. Kwatra, speaking at the 2024 Skin of Color Update. While his comments were focused primarily on individuals with darker skin, his major take home messages were broadly relevant across skin types.
He acknowledged that for years he “had not given seborrheic dermatitis the respect that it deserves” even though this condition comes after only acne and eczema as chief complaints among Black individuals seeing a dermatologist. The estimated global incidence is 5%, according to Dr. Kwatra, but he considers this estimate of an often “forgotten disease” too low.
One reason is that many individuals self-treat with over-the-counter solutions and never bring the complaint to a clinician. Dr. Kwatra said that he now looks for it routinely and points it out to patients who have come to him for another reason.
In patients with darker skin, the signs of SD can differ. While scalp involvement is generally easy to identify across skin types, the inflammation and erythema, sebum production, scaling and itch, and Malassezia that accompanies and drives SD might be missed in a patient with darker skin without specifically looking for these signs.
Skin and Gut Microbiome Involvement Suspected
The underlying causes of SD are understood as an inflammatory process involving keratinocyte disruption and proliferation that ultimately impairs skin barrier function, causes water loss, and produces scale stemming from stratum corneum, but Dr. Kwatra said that there is increasing evidence of a major role for both the skin and gut microbiome.
In regard to the skin microbiome, Malassezia has long been recognized as linked to SD and is a target of treatment, but evidence that the gut microbiome might be participating is relatively new. One clue comes from the fact that oral antifungal therapies, such as itraconazole, are known to reduce risk for SD relapse, an effect that might be a function of their ability to modulate the gut microbiome, according to Dr. Kwatra.
Topical roflumilast, a phosphodiesterase-4 inhibitor, was effective for SD in a vehicle-controlled phase 3 trial published in 2023. He characterized the adverse event profile as “pretty clean,” but he emphasized that a role for many other strategies remains. This is particularly true for challenging forms of SD. For example, topical tacrolimus provided meaningful protection against relapse over a period of more than 6 months in a 2021 trial that enrolled patients with severe facial SD.
The topical Janus kinase inhibitor ruxolitinib, 1.5%, (approved for atopic dermatitis and vitiligo) has also been reported to be effective for refractory facial SD. It is being evaluated in a phase 2 study of SD, according to Dr. Kwatra. A topical PDE4 inhibitor is also being evaluated for SD in a phase 2 study, he said.
Given the heterogeneity of the presentation of SD and the value of combining different mechanisms of action, Dr. Kwatra does not think any drug by itself will be a cure for SD. However, the chances of success with current drug combinations are high.
It is for this reason that Dr. Kwatra encourages clinicians to look for this disease routinely, including among patients who have a different presenting complaint. “Patients do not always bring it up, so bring it up,” he said.
This is good advice, according to Andrew F. Alexis, MD, MPH, professor of clinical dermatology and Vice-chair for Diversity and Inclusion of the Department of Dermatology, Weill Cornell Medicine, New York City. He agreed that the recent introduction of a therapy approved by the FDA is an impetus to look for SD and to talk with patients about treatment options.
In addition, while he also considers roflumilast foam to be a first-line drug, he agreed that combination therapies might be needed to increase the likely of rapid control of scalp and skin involvement. “SD is probably underestimated as a clinical problem, and we do have good treatments to offer for the patients who are affected,” he said at the meeting.
Dr. Kwatra reported no relevant disclosures. Dr. Alexis reported financial relationships with more than 25 pharmaceutical companies.
A version of this article appeared on Medscape.com.
NEW YORK — not only in this group but also overall, now that there is an approved therapy with an array of alternatives and adjunctive medications, according to Shawn Kwatra, MD.
The list of therapies effective against SD, often employed in combination, is lengthy, but topical 0.3% roflumilast foam (Zoryve), approved by the Food and Drug Administration (FDA) late last year for treating SD, has a high rate of efficacy and should now be considered a first-line treatment option, according to Dr. Kwatra, professor and chair of the Department of Dermatology, University of Maryland School of Medicine, Baltimore.
New Approved Therapy Draws Attention to SD
Emphasizing that topical roflumilast does not necessarily replace the use of over-the-counter shampoos and emollients or a list of prescription drugs used off-label to control this condition, he said it is also important for another reason.
“It shines a light on this disease,” said Dr. Kwatra, speaking at the 2024 Skin of Color Update. While his comments were focused primarily on individuals with darker skin, his major take home messages were broadly relevant across skin types.
He acknowledged that for years he “had not given seborrheic dermatitis the respect that it deserves” even though this condition comes after only acne and eczema as chief complaints among Black individuals seeing a dermatologist. The estimated global incidence is 5%, according to Dr. Kwatra, but he considers this estimate of an often “forgotten disease” too low.
One reason is that many individuals self-treat with over-the-counter solutions and never bring the complaint to a clinician. Dr. Kwatra said that he now looks for it routinely and points it out to patients who have come to him for another reason.
In patients with darker skin, the signs of SD can differ. While scalp involvement is generally easy to identify across skin types, the inflammation and erythema, sebum production, scaling and itch, and Malassezia that accompanies and drives SD might be missed in a patient with darker skin without specifically looking for these signs.
Skin and Gut Microbiome Involvement Suspected
The underlying causes of SD are understood as an inflammatory process involving keratinocyte disruption and proliferation that ultimately impairs skin barrier function, causes water loss, and produces scale stemming from stratum corneum, but Dr. Kwatra said that there is increasing evidence of a major role for both the skin and gut microbiome.
In regard to the skin microbiome, Malassezia has long been recognized as linked to SD and is a target of treatment, but evidence that the gut microbiome might be participating is relatively new. One clue comes from the fact that oral antifungal therapies, such as itraconazole, are known to reduce risk for SD relapse, an effect that might be a function of their ability to modulate the gut microbiome, according to Dr. Kwatra.
Topical roflumilast, a phosphodiesterase-4 inhibitor, was effective for SD in a vehicle-controlled phase 3 trial published in 2023. He characterized the adverse event profile as “pretty clean,” but he emphasized that a role for many other strategies remains. This is particularly true for challenging forms of SD. For example, topical tacrolimus provided meaningful protection against relapse over a period of more than 6 months in a 2021 trial that enrolled patients with severe facial SD.
The topical Janus kinase inhibitor ruxolitinib, 1.5%, (approved for atopic dermatitis and vitiligo) has also been reported to be effective for refractory facial SD. It is being evaluated in a phase 2 study of SD, according to Dr. Kwatra. A topical PDE4 inhibitor is also being evaluated for SD in a phase 2 study, he said.
Given the heterogeneity of the presentation of SD and the value of combining different mechanisms of action, Dr. Kwatra does not think any drug by itself will be a cure for SD. However, the chances of success with current drug combinations are high.
It is for this reason that Dr. Kwatra encourages clinicians to look for this disease routinely, including among patients who have a different presenting complaint. “Patients do not always bring it up, so bring it up,” he said.
This is good advice, according to Andrew F. Alexis, MD, MPH, professor of clinical dermatology and Vice-chair for Diversity and Inclusion of the Department of Dermatology, Weill Cornell Medicine, New York City. He agreed that the recent introduction of a therapy approved by the FDA is an impetus to look for SD and to talk with patients about treatment options.
In addition, while he also considers roflumilast foam to be a first-line drug, he agreed that combination therapies might be needed to increase the likely of rapid control of scalp and skin involvement. “SD is probably underestimated as a clinical problem, and we do have good treatments to offer for the patients who are affected,” he said at the meeting.
Dr. Kwatra reported no relevant disclosures. Dr. Alexis reported financial relationships with more than 25 pharmaceutical companies.
A version of this article appeared on Medscape.com.
NEW YORK — not only in this group but also overall, now that there is an approved therapy with an array of alternatives and adjunctive medications, according to Shawn Kwatra, MD.
The list of therapies effective against SD, often employed in combination, is lengthy, but topical 0.3% roflumilast foam (Zoryve), approved by the Food and Drug Administration (FDA) late last year for treating SD, has a high rate of efficacy and should now be considered a first-line treatment option, according to Dr. Kwatra, professor and chair of the Department of Dermatology, University of Maryland School of Medicine, Baltimore.
New Approved Therapy Draws Attention to SD
Emphasizing that topical roflumilast does not necessarily replace the use of over-the-counter shampoos and emollients or a list of prescription drugs used off-label to control this condition, he said it is also important for another reason.
“It shines a light on this disease,” said Dr. Kwatra, speaking at the 2024 Skin of Color Update. While his comments were focused primarily on individuals with darker skin, his major take home messages were broadly relevant across skin types.
He acknowledged that for years he “had not given seborrheic dermatitis the respect that it deserves” even though this condition comes after only acne and eczema as chief complaints among Black individuals seeing a dermatologist. The estimated global incidence is 5%, according to Dr. Kwatra, but he considers this estimate of an often “forgotten disease” too low.
One reason is that many individuals self-treat with over-the-counter solutions and never bring the complaint to a clinician. Dr. Kwatra said that he now looks for it routinely and points it out to patients who have come to him for another reason.
In patients with darker skin, the signs of SD can differ. While scalp involvement is generally easy to identify across skin types, the inflammation and erythema, sebum production, scaling and itch, and Malassezia that accompanies and drives SD might be missed in a patient with darker skin without specifically looking for these signs.
Skin and Gut Microbiome Involvement Suspected
The underlying causes of SD are understood as an inflammatory process involving keratinocyte disruption and proliferation that ultimately impairs skin barrier function, causes water loss, and produces scale stemming from stratum corneum, but Dr. Kwatra said that there is increasing evidence of a major role for both the skin and gut microbiome.
In regard to the skin microbiome, Malassezia has long been recognized as linked to SD and is a target of treatment, but evidence that the gut microbiome might be participating is relatively new. One clue comes from the fact that oral antifungal therapies, such as itraconazole, are known to reduce risk for SD relapse, an effect that might be a function of their ability to modulate the gut microbiome, according to Dr. Kwatra.
Topical roflumilast, a phosphodiesterase-4 inhibitor, was effective for SD in a vehicle-controlled phase 3 trial published in 2023. He characterized the adverse event profile as “pretty clean,” but he emphasized that a role for many other strategies remains. This is particularly true for challenging forms of SD. For example, topical tacrolimus provided meaningful protection against relapse over a period of more than 6 months in a 2021 trial that enrolled patients with severe facial SD.
The topical Janus kinase inhibitor ruxolitinib, 1.5%, (approved for atopic dermatitis and vitiligo) has also been reported to be effective for refractory facial SD. It is being evaluated in a phase 2 study of SD, according to Dr. Kwatra. A topical PDE4 inhibitor is also being evaluated for SD in a phase 2 study, he said.
Given the heterogeneity of the presentation of SD and the value of combining different mechanisms of action, Dr. Kwatra does not think any drug by itself will be a cure for SD. However, the chances of success with current drug combinations are high.
It is for this reason that Dr. Kwatra encourages clinicians to look for this disease routinely, including among patients who have a different presenting complaint. “Patients do not always bring it up, so bring it up,” he said.
This is good advice, according to Andrew F. Alexis, MD, MPH, professor of clinical dermatology and Vice-chair for Diversity and Inclusion of the Department of Dermatology, Weill Cornell Medicine, New York City. He agreed that the recent introduction of a therapy approved by the FDA is an impetus to look for SD and to talk with patients about treatment options.
In addition, while he also considers roflumilast foam to be a first-line drug, he agreed that combination therapies might be needed to increase the likely of rapid control of scalp and skin involvement. “SD is probably underestimated as a clinical problem, and we do have good treatments to offer for the patients who are affected,” he said at the meeting.
Dr. Kwatra reported no relevant disclosures. Dr. Alexis reported financial relationships with more than 25 pharmaceutical companies.
A version of this article appeared on Medscape.com.
FROM SOC 2024
Identifying Drug-Induced Rashes in Skin of Color: Heightened Awareness Can Accelerate Diagnosis
NEW YORK — Because of their heterogeneity in appearance, to speed the diagnosis.
This risk for a delayed or missed diagnosis in patients with darker skin is shared across skin rashes, but drug-induced hypersensitivity syndrome (DIHS) is a telling example, according to Joanna Harp, MD, director of the Inpatient Dermatology Consult Service, NewYork–Presbyterian Hospital, New York City.
DIHS, also known as a drug reaction with eosinophilia and systemic symptoms, is a type IV hypersensitivity reaction, Dr. Harp explained. While the fact that this disorder does not always include eosinophilia prompted the DIHS acronym, the maculopapular rash often serves as a critical clue of the underlying etiology.
In patients with darker skin, DIHS skin manifestations “can look different, can be more severe, and can have worse outcomes,” Dr. Harp said. As with other skin rashes that are primarily erythematous, the DIHS rash is often more subtle in Black-skinned patients, typically appearing gray or violaceous rather than red.
“The high amount of scale can be a clue,” said Dr. Harp, speaking at the 2024 Skin of Color Update. Scale is particularly prominent among Black patients, she said, because of the greater relative transepidermal water loss than lighter skin, increasing dryness and susceptibility to scale.
The maculopapular rash is “similar to a simple drug eruption, although it is usually more impressive,” she said. Emphasizing that DIHS is a systemic disease, she noted that the characteristic rash is typically accompanied by inflammation in multiple organs that not only includes the mucous membranes but can include major organs such as the lungs, kidneys, and heart.
In patients with DIHS and many of the even more serious types of rashes traced to drug exposures, such as Stevens-Johnson syndrome (SJS) or erythema multiforme, the delay to appearance of the rash from the time of exposure can be the most confusing element.
“It can be months for some drugs such as allopurinol,” said Dr. Harp, pointing out that Black and Asian patients are more likely to carry the HLA-B*5801 genotype, a known risk factor for allopurinol hypersensitivity.
Signs of AGEP Can Be Subtle in Black Patients
Some of the same principles for diagnosing drug-induced rash in darker skin can also be applied to acute generalized exanthematous pustulosis (AGEP), another type IV hypersensitivity reaction. Like all drug-induced rashes, the earlier AGEP is recognized and treated, the better the outcome, but in Black patients, the signs can be subtle.
“The onset is usually fast and occurs in 1-2 days after [the causative drug] exposure,” said Dr. Harp, adding that antibiotics, such as cephalosporins or penicillin, and calcium channel blockers are among the prominent causes of AGEP.
One of the hallmark signs of early-onset AGEP are tiny erythematous pustules in flexural areas, such as the neck or the armpits. The issue of detecting erythema in darker skin is also relevant to this area, but there is an additional problem, according to Dr. Harp. The pustules often dry up quickly, leaving a neutrophilic scale that further complicates the effort to see the characteristic erythema.
“If you see a lot of scale, look for erythema underneath. Think of inflammation,” Dr. Harp said, explaining that the clinical appearance evolves quickly. “If you do not see the pustules, it does not mean they were not there; you just missed them.”
In addition to the flexural areas, “AGEP loves the ears, the face, and the geographic tongue,” she said, offering several pearls to help with the diagnosis. These include side lighting to make papules easier to see, pressing on the skin to highlight the difference between erythematous skin and blanched skin, and checking less pigmented skin, such as on the hands and feet, which makes erythema easier to see.
Steroids are often the first-line treatment for drug-induced skin rashes, but Dr. Harp moves to etanercept or cyclosporine for the most serious drug reactions, such as SJS and toxic epidermal necrolysis.
Etanercept is typically her first choice because patients with systemic hypersensitivity reactions with major organ involvement are often quite ill, making cyclosporine harder to use. In her experience, etanercept has been well tolerated.
Conversely, she cautioned against the use of intravenous immunoglobulin (IVIG). Although this has been used traditionally for severe drug hypersensitivity reactions, “the data are not there,” she said. The data are stronger for a combination of high-dose steroids and IVIG, but she thinks even these data are inconsistent and not as strong as the data supporting etanercept or cyclosporine. She encouraged centers still using IVIG to consider alternatives.
After drug sensitivity reactions are controlled, follow-up care is particularly important for Black patients who face greater risks for sequelae, such as hypopigmentation, hyperpigmentation, or keloids. She recommended aggressive use of emollients and sunscreens for an extended period after lesions resolve to lessen these risks.
Differences in the manifestations of drug-induced skin rashes by race and ethnicity are important and perhaps underappreciated, agreed Shawn Kwatra, MD, professor and chairman of the Department of Dermatology, University of Maryland, Baltimore.
Asked to comment at the meeting, Dr. Kwatra said that he appreciated Dr. Harp’s effort to translate published data and her experience into an overview that increases awareness of the risk for missed or delayed diagnoses of drug-induced rashes in skin of color. He noted that the strategies to identify erythema and pustules, such as increased suspicion in skin of color and the extra steps to rule them out, such as the use of side lighting in the case of pustules for AGEP, are simple and practical.
Dr. Harp and Dr. Kwatra had no relevant disclosures.
A version of this article appeared on Medscape.com.
NEW YORK — Because of their heterogeneity in appearance, to speed the diagnosis.
This risk for a delayed or missed diagnosis in patients with darker skin is shared across skin rashes, but drug-induced hypersensitivity syndrome (DIHS) is a telling example, according to Joanna Harp, MD, director of the Inpatient Dermatology Consult Service, NewYork–Presbyterian Hospital, New York City.
DIHS, also known as a drug reaction with eosinophilia and systemic symptoms, is a type IV hypersensitivity reaction, Dr. Harp explained. While the fact that this disorder does not always include eosinophilia prompted the DIHS acronym, the maculopapular rash often serves as a critical clue of the underlying etiology.
In patients with darker skin, DIHS skin manifestations “can look different, can be more severe, and can have worse outcomes,” Dr. Harp said. As with other skin rashes that are primarily erythematous, the DIHS rash is often more subtle in Black-skinned patients, typically appearing gray or violaceous rather than red.
“The high amount of scale can be a clue,” said Dr. Harp, speaking at the 2024 Skin of Color Update. Scale is particularly prominent among Black patients, she said, because of the greater relative transepidermal water loss than lighter skin, increasing dryness and susceptibility to scale.
The maculopapular rash is “similar to a simple drug eruption, although it is usually more impressive,” she said. Emphasizing that DIHS is a systemic disease, she noted that the characteristic rash is typically accompanied by inflammation in multiple organs that not only includes the mucous membranes but can include major organs such as the lungs, kidneys, and heart.
In patients with DIHS and many of the even more serious types of rashes traced to drug exposures, such as Stevens-Johnson syndrome (SJS) or erythema multiforme, the delay to appearance of the rash from the time of exposure can be the most confusing element.
“It can be months for some drugs such as allopurinol,” said Dr. Harp, pointing out that Black and Asian patients are more likely to carry the HLA-B*5801 genotype, a known risk factor for allopurinol hypersensitivity.
Signs of AGEP Can Be Subtle in Black Patients
Some of the same principles for diagnosing drug-induced rash in darker skin can also be applied to acute generalized exanthematous pustulosis (AGEP), another type IV hypersensitivity reaction. Like all drug-induced rashes, the earlier AGEP is recognized and treated, the better the outcome, but in Black patients, the signs can be subtle.
“The onset is usually fast and occurs in 1-2 days after [the causative drug] exposure,” said Dr. Harp, adding that antibiotics, such as cephalosporins or penicillin, and calcium channel blockers are among the prominent causes of AGEP.
One of the hallmark signs of early-onset AGEP are tiny erythematous pustules in flexural areas, such as the neck or the armpits. The issue of detecting erythema in darker skin is also relevant to this area, but there is an additional problem, according to Dr. Harp. The pustules often dry up quickly, leaving a neutrophilic scale that further complicates the effort to see the characteristic erythema.
“If you see a lot of scale, look for erythema underneath. Think of inflammation,” Dr. Harp said, explaining that the clinical appearance evolves quickly. “If you do not see the pustules, it does not mean they were not there; you just missed them.”
In addition to the flexural areas, “AGEP loves the ears, the face, and the geographic tongue,” she said, offering several pearls to help with the diagnosis. These include side lighting to make papules easier to see, pressing on the skin to highlight the difference between erythematous skin and blanched skin, and checking less pigmented skin, such as on the hands and feet, which makes erythema easier to see.
Steroids are often the first-line treatment for drug-induced skin rashes, but Dr. Harp moves to etanercept or cyclosporine for the most serious drug reactions, such as SJS and toxic epidermal necrolysis.
Etanercept is typically her first choice because patients with systemic hypersensitivity reactions with major organ involvement are often quite ill, making cyclosporine harder to use. In her experience, etanercept has been well tolerated.
Conversely, she cautioned against the use of intravenous immunoglobulin (IVIG). Although this has been used traditionally for severe drug hypersensitivity reactions, “the data are not there,” she said. The data are stronger for a combination of high-dose steroids and IVIG, but she thinks even these data are inconsistent and not as strong as the data supporting etanercept or cyclosporine. She encouraged centers still using IVIG to consider alternatives.
After drug sensitivity reactions are controlled, follow-up care is particularly important for Black patients who face greater risks for sequelae, such as hypopigmentation, hyperpigmentation, or keloids. She recommended aggressive use of emollients and sunscreens for an extended period after lesions resolve to lessen these risks.
Differences in the manifestations of drug-induced skin rashes by race and ethnicity are important and perhaps underappreciated, agreed Shawn Kwatra, MD, professor and chairman of the Department of Dermatology, University of Maryland, Baltimore.
Asked to comment at the meeting, Dr. Kwatra said that he appreciated Dr. Harp’s effort to translate published data and her experience into an overview that increases awareness of the risk for missed or delayed diagnoses of drug-induced rashes in skin of color. He noted that the strategies to identify erythema and pustules, such as increased suspicion in skin of color and the extra steps to rule them out, such as the use of side lighting in the case of pustules for AGEP, are simple and practical.
Dr. Harp and Dr. Kwatra had no relevant disclosures.
A version of this article appeared on Medscape.com.
NEW YORK — Because of their heterogeneity in appearance, to speed the diagnosis.
This risk for a delayed or missed diagnosis in patients with darker skin is shared across skin rashes, but drug-induced hypersensitivity syndrome (DIHS) is a telling example, according to Joanna Harp, MD, director of the Inpatient Dermatology Consult Service, NewYork–Presbyterian Hospital, New York City.
DIHS, also known as a drug reaction with eosinophilia and systemic symptoms, is a type IV hypersensitivity reaction, Dr. Harp explained. While the fact that this disorder does not always include eosinophilia prompted the DIHS acronym, the maculopapular rash often serves as a critical clue of the underlying etiology.
In patients with darker skin, DIHS skin manifestations “can look different, can be more severe, and can have worse outcomes,” Dr. Harp said. As with other skin rashes that are primarily erythematous, the DIHS rash is often more subtle in Black-skinned patients, typically appearing gray or violaceous rather than red.
“The high amount of scale can be a clue,” said Dr. Harp, speaking at the 2024 Skin of Color Update. Scale is particularly prominent among Black patients, she said, because of the greater relative transepidermal water loss than lighter skin, increasing dryness and susceptibility to scale.
The maculopapular rash is “similar to a simple drug eruption, although it is usually more impressive,” she said. Emphasizing that DIHS is a systemic disease, she noted that the characteristic rash is typically accompanied by inflammation in multiple organs that not only includes the mucous membranes but can include major organs such as the lungs, kidneys, and heart.
In patients with DIHS and many of the even more serious types of rashes traced to drug exposures, such as Stevens-Johnson syndrome (SJS) or erythema multiforme, the delay to appearance of the rash from the time of exposure can be the most confusing element.
“It can be months for some drugs such as allopurinol,” said Dr. Harp, pointing out that Black and Asian patients are more likely to carry the HLA-B*5801 genotype, a known risk factor for allopurinol hypersensitivity.
Signs of AGEP Can Be Subtle in Black Patients
Some of the same principles for diagnosing drug-induced rash in darker skin can also be applied to acute generalized exanthematous pustulosis (AGEP), another type IV hypersensitivity reaction. Like all drug-induced rashes, the earlier AGEP is recognized and treated, the better the outcome, but in Black patients, the signs can be subtle.
“The onset is usually fast and occurs in 1-2 days after [the causative drug] exposure,” said Dr. Harp, adding that antibiotics, such as cephalosporins or penicillin, and calcium channel blockers are among the prominent causes of AGEP.
One of the hallmark signs of early-onset AGEP are tiny erythematous pustules in flexural areas, such as the neck or the armpits. The issue of detecting erythema in darker skin is also relevant to this area, but there is an additional problem, according to Dr. Harp. The pustules often dry up quickly, leaving a neutrophilic scale that further complicates the effort to see the characteristic erythema.
“If you see a lot of scale, look for erythema underneath. Think of inflammation,” Dr. Harp said, explaining that the clinical appearance evolves quickly. “If you do not see the pustules, it does not mean they were not there; you just missed them.”
In addition to the flexural areas, “AGEP loves the ears, the face, and the geographic tongue,” she said, offering several pearls to help with the diagnosis. These include side lighting to make papules easier to see, pressing on the skin to highlight the difference between erythematous skin and blanched skin, and checking less pigmented skin, such as on the hands and feet, which makes erythema easier to see.
Steroids are often the first-line treatment for drug-induced skin rashes, but Dr. Harp moves to etanercept or cyclosporine for the most serious drug reactions, such as SJS and toxic epidermal necrolysis.
Etanercept is typically her first choice because patients with systemic hypersensitivity reactions with major organ involvement are often quite ill, making cyclosporine harder to use. In her experience, etanercept has been well tolerated.
Conversely, she cautioned against the use of intravenous immunoglobulin (IVIG). Although this has been used traditionally for severe drug hypersensitivity reactions, “the data are not there,” she said. The data are stronger for a combination of high-dose steroids and IVIG, but she thinks even these data are inconsistent and not as strong as the data supporting etanercept or cyclosporine. She encouraged centers still using IVIG to consider alternatives.
After drug sensitivity reactions are controlled, follow-up care is particularly important for Black patients who face greater risks for sequelae, such as hypopigmentation, hyperpigmentation, or keloids. She recommended aggressive use of emollients and sunscreens for an extended period after lesions resolve to lessen these risks.
Differences in the manifestations of drug-induced skin rashes by race and ethnicity are important and perhaps underappreciated, agreed Shawn Kwatra, MD, professor and chairman of the Department of Dermatology, University of Maryland, Baltimore.
Asked to comment at the meeting, Dr. Kwatra said that he appreciated Dr. Harp’s effort to translate published data and her experience into an overview that increases awareness of the risk for missed or delayed diagnoses of drug-induced rashes in skin of color. He noted that the strategies to identify erythema and pustules, such as increased suspicion in skin of color and the extra steps to rule them out, such as the use of side lighting in the case of pustules for AGEP, are simple and practical.
Dr. Harp and Dr. Kwatra had no relevant disclosures.
A version of this article appeared on Medscape.com.
FROM SOC 2024
FDA Initiative Aims to Improve Diversity in Clinical Trials
NEW YORK — Underrepresentation by gender and race in major clinical trials has been a cause for complaint for decades, but the Food and Drug Administration (FDA) has drafted a regulatory solution to this issue expected to be implemented sometime in 2025.
This initiative, known as the according to Valerie M. Harvey, MD, MPH, associate clinical professor, Edward Via College of Osteopathic Medicine, Blacksburg, Virginia. These rules will be codified, she said at the 2024 Skin of Color Update.
Once the DAP is enacted, “the sponsor must specify the rationale and goals for study enrollment by age, ethnicity, sex, and race,” she said. Furthermore, the submission to the FDA must “describe the methods to meet the diversity benchmarks.”
Lack of Trial Diversity Is Common Across Medicine
Although she focused on the relevance of this initiative to dermatology, Dr. Harvey said the lack of diversity in clinical trials is pervasive throughout medicine. In one survey of randomized controlled trials, less than 60% of trials even specified the race and ethnicity of the participants. In recent psoriasis trials, only 30% met a diversity definition of ≥ 20% of patients identifying as minority (Black, Hispanic, Asian, or other non-White group), said Dr. Harvey, who practices dermatology in Newport News, Virginia.
The FDA draft guidance for the DAP was released in June 2024 and is now available for submitting comments (until September 26). The plan is expected to be published in June 2025, according to Dr. Harvey. It will pertain to all pivotal and phase 3 trials enrolling 180 days after the publication date and will be relevant to all drugs and biologics as well as certain devices.
This initiative could be a critical step toward ensuring diversity in major clinical trials after years of stagnation, Dr. Harvey said, noting that despite repeated calls for more diversity in clinical trials, the literature suggests “little progress.”
However, she said that increasing diversity in clinical trials is just one step toward gathering data about the generalizability of efficacy and safety across racial and ethnic groups. A much more complex issue involves how race and ethnicity are defined in order to understand differences, if any, for efficacy and risk.
“Race is a dynamic social construct and a poor measure for biologic variation and skin color,” Dr. Harvey said. This means that work is needed to address the more complex issue of race and ethnicity stratification that will help clinicians understand the relative benefits and risks for the drugs in these trials.
Rather than differences based on genetic or other sources of biologic differences, she said, outcomes by race alone are often suspected of reflecting disparities in access to healthcare rather than a difference in therapeutic response.
Skin Color Is Inadequate to Define Race
When stratifying patients by race or ethnicity, Dr. Harvey said that “we have to be very, very careful in considering the study purpose and what the study question is.” A study attempting to compare benefits and risks among subgroups by race or ethnicity will require descriptors beyond skin color.
The recognized limitations of measuring skin tone as a surrogate of race are one reason for widespread interest in moving away from the Fitzpatrick skin type (FST) rating that has been widely considered a standard, according to Dr. Harvey. Several alternatives have been proposed, including the Monk Skin Tone Scale, the Individual Typology Angle, and the Eumelanin Human Skin Color Scale, but she cautioned that these are less well validated and generally have the limitations of the FST.
If skin color was ever useful for grouping individuals on the basis of shared physiology, growing rates of intermarriage and immigration have made skin color increasingly irrelevant to racial identity. If the goal is to evaluate the safety and efficacy of drugs across racial groups and ethnicities, the characterization of populations will almost certainly require multiple descriptors and biomarkers, she said.
“It is very important to have many tools for characterizing patients by skin type,” Susan Taylor, MD, professor of dermatology and vice chair for diversity, equity, and inclusion for the Department of Dermatology, University of Pennsylvania, Philadelphia, said in an interview at the meeting.
The reason is “there are limitations to all of them,” she said, noting also that the questions being asked about how and if skin color and race are relevant to therapeutic options differ by the question, such as innate response or access to care.
Dr. Taylor is part of a workshop that she said is evaluating a combination of instruments for characterizing skin color and race in ways relevant to the specific question being asked.
The solutions might differ. While simple clinical assessments involving skin color might be made with methods captured on a smartphone app, Dr. Taylor acknowledged that far more complex tools might be required to document the effect of racial or ethnic differences in drug efficacy and safety in a research setting.
Outside of a research setting, any tools that might be useful for assessing race as a variable must be practical, according to Dr. Harvey. She suggested that these must be time efficient, of reasonable cost, and most importantly, reliable.
Tools meeting these criteria do not currently exist, but Dr. Harvey said the work is underway. She expects a “top-down” collaborative approach to validate alternatives to the FST. If such tools can be developed with buy-in from the FDA, they might be particularly useful for translating trial data to patient care, she added.
Dr. Harvey reported financial relationships with AbbVie, Bristol-Myers Squibb, Janssen, Johnson & Johnson, L’Oréal, and SkinCeuticals. Dr. Taylor, president-elect of the American Academy of Dermatology, reported financial relationships with more than 25 pharmaceutical and cosmetic companies.
A version of this article appeared on Medscape.com.
NEW YORK — Underrepresentation by gender and race in major clinical trials has been a cause for complaint for decades, but the Food and Drug Administration (FDA) has drafted a regulatory solution to this issue expected to be implemented sometime in 2025.
This initiative, known as the according to Valerie M. Harvey, MD, MPH, associate clinical professor, Edward Via College of Osteopathic Medicine, Blacksburg, Virginia. These rules will be codified, she said at the 2024 Skin of Color Update.
Once the DAP is enacted, “the sponsor must specify the rationale and goals for study enrollment by age, ethnicity, sex, and race,” she said. Furthermore, the submission to the FDA must “describe the methods to meet the diversity benchmarks.”
Lack of Trial Diversity Is Common Across Medicine
Although she focused on the relevance of this initiative to dermatology, Dr. Harvey said the lack of diversity in clinical trials is pervasive throughout medicine. In one survey of randomized controlled trials, less than 60% of trials even specified the race and ethnicity of the participants. In recent psoriasis trials, only 30% met a diversity definition of ≥ 20% of patients identifying as minority (Black, Hispanic, Asian, or other non-White group), said Dr. Harvey, who practices dermatology in Newport News, Virginia.
The FDA draft guidance for the DAP was released in June 2024 and is now available for submitting comments (until September 26). The plan is expected to be published in June 2025, according to Dr. Harvey. It will pertain to all pivotal and phase 3 trials enrolling 180 days after the publication date and will be relevant to all drugs and biologics as well as certain devices.
This initiative could be a critical step toward ensuring diversity in major clinical trials after years of stagnation, Dr. Harvey said, noting that despite repeated calls for more diversity in clinical trials, the literature suggests “little progress.”
However, she said that increasing diversity in clinical trials is just one step toward gathering data about the generalizability of efficacy and safety across racial and ethnic groups. A much more complex issue involves how race and ethnicity are defined in order to understand differences, if any, for efficacy and risk.
“Race is a dynamic social construct and a poor measure for biologic variation and skin color,” Dr. Harvey said. This means that work is needed to address the more complex issue of race and ethnicity stratification that will help clinicians understand the relative benefits and risks for the drugs in these trials.
Rather than differences based on genetic or other sources of biologic differences, she said, outcomes by race alone are often suspected of reflecting disparities in access to healthcare rather than a difference in therapeutic response.
Skin Color Is Inadequate to Define Race
When stratifying patients by race or ethnicity, Dr. Harvey said that “we have to be very, very careful in considering the study purpose and what the study question is.” A study attempting to compare benefits and risks among subgroups by race or ethnicity will require descriptors beyond skin color.
The recognized limitations of measuring skin tone as a surrogate of race are one reason for widespread interest in moving away from the Fitzpatrick skin type (FST) rating that has been widely considered a standard, according to Dr. Harvey. Several alternatives have been proposed, including the Monk Skin Tone Scale, the Individual Typology Angle, and the Eumelanin Human Skin Color Scale, but she cautioned that these are less well validated and generally have the limitations of the FST.
If skin color was ever useful for grouping individuals on the basis of shared physiology, growing rates of intermarriage and immigration have made skin color increasingly irrelevant to racial identity. If the goal is to evaluate the safety and efficacy of drugs across racial groups and ethnicities, the characterization of populations will almost certainly require multiple descriptors and biomarkers, she said.
“It is very important to have many tools for characterizing patients by skin type,” Susan Taylor, MD, professor of dermatology and vice chair for diversity, equity, and inclusion for the Department of Dermatology, University of Pennsylvania, Philadelphia, said in an interview at the meeting.
The reason is “there are limitations to all of them,” she said, noting also that the questions being asked about how and if skin color and race are relevant to therapeutic options differ by the question, such as innate response or access to care.
Dr. Taylor is part of a workshop that she said is evaluating a combination of instruments for characterizing skin color and race in ways relevant to the specific question being asked.
The solutions might differ. While simple clinical assessments involving skin color might be made with methods captured on a smartphone app, Dr. Taylor acknowledged that far more complex tools might be required to document the effect of racial or ethnic differences in drug efficacy and safety in a research setting.
Outside of a research setting, any tools that might be useful for assessing race as a variable must be practical, according to Dr. Harvey. She suggested that these must be time efficient, of reasonable cost, and most importantly, reliable.
Tools meeting these criteria do not currently exist, but Dr. Harvey said the work is underway. She expects a “top-down” collaborative approach to validate alternatives to the FST. If such tools can be developed with buy-in from the FDA, they might be particularly useful for translating trial data to patient care, she added.
Dr. Harvey reported financial relationships with AbbVie, Bristol-Myers Squibb, Janssen, Johnson & Johnson, L’Oréal, and SkinCeuticals. Dr. Taylor, president-elect of the American Academy of Dermatology, reported financial relationships with more than 25 pharmaceutical and cosmetic companies.
A version of this article appeared on Medscape.com.
NEW YORK — Underrepresentation by gender and race in major clinical trials has been a cause for complaint for decades, but the Food and Drug Administration (FDA) has drafted a regulatory solution to this issue expected to be implemented sometime in 2025.
This initiative, known as the according to Valerie M. Harvey, MD, MPH, associate clinical professor, Edward Via College of Osteopathic Medicine, Blacksburg, Virginia. These rules will be codified, she said at the 2024 Skin of Color Update.
Once the DAP is enacted, “the sponsor must specify the rationale and goals for study enrollment by age, ethnicity, sex, and race,” she said. Furthermore, the submission to the FDA must “describe the methods to meet the diversity benchmarks.”
Lack of Trial Diversity Is Common Across Medicine
Although she focused on the relevance of this initiative to dermatology, Dr. Harvey said the lack of diversity in clinical trials is pervasive throughout medicine. In one survey of randomized controlled trials, less than 60% of trials even specified the race and ethnicity of the participants. In recent psoriasis trials, only 30% met a diversity definition of ≥ 20% of patients identifying as minority (Black, Hispanic, Asian, or other non-White group), said Dr. Harvey, who practices dermatology in Newport News, Virginia.
The FDA draft guidance for the DAP was released in June 2024 and is now available for submitting comments (until September 26). The plan is expected to be published in June 2025, according to Dr. Harvey. It will pertain to all pivotal and phase 3 trials enrolling 180 days after the publication date and will be relevant to all drugs and biologics as well as certain devices.
This initiative could be a critical step toward ensuring diversity in major clinical trials after years of stagnation, Dr. Harvey said, noting that despite repeated calls for more diversity in clinical trials, the literature suggests “little progress.”
However, she said that increasing diversity in clinical trials is just one step toward gathering data about the generalizability of efficacy and safety across racial and ethnic groups. A much more complex issue involves how race and ethnicity are defined in order to understand differences, if any, for efficacy and risk.
“Race is a dynamic social construct and a poor measure for biologic variation and skin color,” Dr. Harvey said. This means that work is needed to address the more complex issue of race and ethnicity stratification that will help clinicians understand the relative benefits and risks for the drugs in these trials.
Rather than differences based on genetic or other sources of biologic differences, she said, outcomes by race alone are often suspected of reflecting disparities in access to healthcare rather than a difference in therapeutic response.
Skin Color Is Inadequate to Define Race
When stratifying patients by race or ethnicity, Dr. Harvey said that “we have to be very, very careful in considering the study purpose and what the study question is.” A study attempting to compare benefits and risks among subgroups by race or ethnicity will require descriptors beyond skin color.
The recognized limitations of measuring skin tone as a surrogate of race are one reason for widespread interest in moving away from the Fitzpatrick skin type (FST) rating that has been widely considered a standard, according to Dr. Harvey. Several alternatives have been proposed, including the Monk Skin Tone Scale, the Individual Typology Angle, and the Eumelanin Human Skin Color Scale, but she cautioned that these are less well validated and generally have the limitations of the FST.
If skin color was ever useful for grouping individuals on the basis of shared physiology, growing rates of intermarriage and immigration have made skin color increasingly irrelevant to racial identity. If the goal is to evaluate the safety and efficacy of drugs across racial groups and ethnicities, the characterization of populations will almost certainly require multiple descriptors and biomarkers, she said.
“It is very important to have many tools for characterizing patients by skin type,” Susan Taylor, MD, professor of dermatology and vice chair for diversity, equity, and inclusion for the Department of Dermatology, University of Pennsylvania, Philadelphia, said in an interview at the meeting.
The reason is “there are limitations to all of them,” she said, noting also that the questions being asked about how and if skin color and race are relevant to therapeutic options differ by the question, such as innate response or access to care.
Dr. Taylor is part of a workshop that she said is evaluating a combination of instruments for characterizing skin color and race in ways relevant to the specific question being asked.
The solutions might differ. While simple clinical assessments involving skin color might be made with methods captured on a smartphone app, Dr. Taylor acknowledged that far more complex tools might be required to document the effect of racial or ethnic differences in drug efficacy and safety in a research setting.
Outside of a research setting, any tools that might be useful for assessing race as a variable must be practical, according to Dr. Harvey. She suggested that these must be time efficient, of reasonable cost, and most importantly, reliable.
Tools meeting these criteria do not currently exist, but Dr. Harvey said the work is underway. She expects a “top-down” collaborative approach to validate alternatives to the FST. If such tools can be developed with buy-in from the FDA, they might be particularly useful for translating trial data to patient care, she added.
Dr. Harvey reported financial relationships with AbbVie, Bristol-Myers Squibb, Janssen, Johnson & Johnson, L’Oréal, and SkinCeuticals. Dr. Taylor, president-elect of the American Academy of Dermatology, reported financial relationships with more than 25 pharmaceutical and cosmetic companies.
A version of this article appeared on Medscape.com.
FROM SOC 2024
Diabetes Drug Improved Symptoms in Small Study of Women With Central Centrifugal Cicatricial Alopecia
TOPLINE:
in a retrospective case series.
METHODOLOGY:
- Researchers conducted a case series involving 12 Black women in their 30s, 40s, and 50s, with biopsy-confirmed, treatment-refractory CCCA, a chronic inflammatory hair disorder characterized by permanent hair loss, from the Johns Hopkins University alopecia clinic.
- Participants received CCCA treatment for at least 6 months and had stagnant or worsening symptoms before oral extended-release metformin (500 mg daily) was added to treatment. (Treatments included topical clobetasol, compounded minoxidil, and platelet-rich plasma injections.)
- Scalp biopsies were collected from four patients before and after metformin treatment to evaluate gene expression changes.
- Changes in clinical symptoms were assessed, including pruritus, inflammation, pain, scalp resistance, and hair regrowth, following initiation of metformin treatment.
TAKEAWAY:
- Metformin led to significant clinical improvement in eight patients, which included reductions in scalp pain, scalp resistance, pruritus, and inflammation. However, two patients experienced worsening symptoms.
- Six patients showed clinical evidence of hair regrowth after at least 6 months of metformin treatment with one experiencing hair loss again 3 months after discontinuing treatment.
- Transcriptomic analysis revealed 34 up-regulated genes, which included up-regulated of 23 hair keratin–associated proteins, and pathways related to keratinization, epidermis development, and the hair cycle. In addition, eight genes were down-regulated, with pathways that included those associated with extracellular matrix organization, collagen fibril organization, and collagen metabolism.
- Gene set variation analysis showed reduced expression of T helper 17 cell and epithelial-mesenchymal transition pathways and elevated adenosine monophosphate kinase signaling and keratin-associated proteins after treatment with metformin.
IN PRACTICE:
“Metformin’s ability to concomitantly target fibrosis and inflammation provides a plausible mechanism for its therapeutic effects in CCCA and other fibrosing alopecia disorders,” the authors concluded. But, they added, “larger prospective, placebo-controlled randomized clinical trials are needed to rigorously evaluate metformin’s efficacy and optimal dosing for treatment of cicatricial alopecias.”
SOURCE:
The study was led by Aaron Bao, Department of Dermatology, Johns Hopkins University School of Medicine, Baltimore, Maryland, and was published online on September 4 in JAMA Dermatology.
LIMITATIONS:
A small sample size, retrospective design, lack of a placebo control group, and the single-center setting limited the generalizability of the study findings. Additionally, the absence of a validated activity or severity scale for CCCA and the single posttreatment sampling limit the assessment and comparison of clinical symptoms and transcriptomic changes.
DISCLOSURES:
The study was supported by the American Academy of Dermatology. One author reported several ties with pharmaceutical companies, a pending patent, and authorship for the UpToDate section on CCCA.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
in a retrospective case series.
METHODOLOGY:
- Researchers conducted a case series involving 12 Black women in their 30s, 40s, and 50s, with biopsy-confirmed, treatment-refractory CCCA, a chronic inflammatory hair disorder characterized by permanent hair loss, from the Johns Hopkins University alopecia clinic.
- Participants received CCCA treatment for at least 6 months and had stagnant or worsening symptoms before oral extended-release metformin (500 mg daily) was added to treatment. (Treatments included topical clobetasol, compounded minoxidil, and platelet-rich plasma injections.)
- Scalp biopsies were collected from four patients before and after metformin treatment to evaluate gene expression changes.
- Changes in clinical symptoms were assessed, including pruritus, inflammation, pain, scalp resistance, and hair regrowth, following initiation of metformin treatment.
TAKEAWAY:
- Metformin led to significant clinical improvement in eight patients, which included reductions in scalp pain, scalp resistance, pruritus, and inflammation. However, two patients experienced worsening symptoms.
- Six patients showed clinical evidence of hair regrowth after at least 6 months of metformin treatment with one experiencing hair loss again 3 months after discontinuing treatment.
- Transcriptomic analysis revealed 34 up-regulated genes, which included up-regulated of 23 hair keratin–associated proteins, and pathways related to keratinization, epidermis development, and the hair cycle. In addition, eight genes were down-regulated, with pathways that included those associated with extracellular matrix organization, collagen fibril organization, and collagen metabolism.
- Gene set variation analysis showed reduced expression of T helper 17 cell and epithelial-mesenchymal transition pathways and elevated adenosine monophosphate kinase signaling and keratin-associated proteins after treatment with metformin.
IN PRACTICE:
“Metformin’s ability to concomitantly target fibrosis and inflammation provides a plausible mechanism for its therapeutic effects in CCCA and other fibrosing alopecia disorders,” the authors concluded. But, they added, “larger prospective, placebo-controlled randomized clinical trials are needed to rigorously evaluate metformin’s efficacy and optimal dosing for treatment of cicatricial alopecias.”
SOURCE:
The study was led by Aaron Bao, Department of Dermatology, Johns Hopkins University School of Medicine, Baltimore, Maryland, and was published online on September 4 in JAMA Dermatology.
LIMITATIONS:
A small sample size, retrospective design, lack of a placebo control group, and the single-center setting limited the generalizability of the study findings. Additionally, the absence of a validated activity or severity scale for CCCA and the single posttreatment sampling limit the assessment and comparison of clinical symptoms and transcriptomic changes.
DISCLOSURES:
The study was supported by the American Academy of Dermatology. One author reported several ties with pharmaceutical companies, a pending patent, and authorship for the UpToDate section on CCCA.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
in a retrospective case series.
METHODOLOGY:
- Researchers conducted a case series involving 12 Black women in their 30s, 40s, and 50s, with biopsy-confirmed, treatment-refractory CCCA, a chronic inflammatory hair disorder characterized by permanent hair loss, from the Johns Hopkins University alopecia clinic.
- Participants received CCCA treatment for at least 6 months and had stagnant or worsening symptoms before oral extended-release metformin (500 mg daily) was added to treatment. (Treatments included topical clobetasol, compounded minoxidil, and platelet-rich plasma injections.)
- Scalp biopsies were collected from four patients before and after metformin treatment to evaluate gene expression changes.
- Changes in clinical symptoms were assessed, including pruritus, inflammation, pain, scalp resistance, and hair regrowth, following initiation of metformin treatment.
TAKEAWAY:
- Metformin led to significant clinical improvement in eight patients, which included reductions in scalp pain, scalp resistance, pruritus, and inflammation. However, two patients experienced worsening symptoms.
- Six patients showed clinical evidence of hair regrowth after at least 6 months of metformin treatment with one experiencing hair loss again 3 months after discontinuing treatment.
- Transcriptomic analysis revealed 34 up-regulated genes, which included up-regulated of 23 hair keratin–associated proteins, and pathways related to keratinization, epidermis development, and the hair cycle. In addition, eight genes were down-regulated, with pathways that included those associated with extracellular matrix organization, collagen fibril organization, and collagen metabolism.
- Gene set variation analysis showed reduced expression of T helper 17 cell and epithelial-mesenchymal transition pathways and elevated adenosine monophosphate kinase signaling and keratin-associated proteins after treatment with metformin.
IN PRACTICE:
“Metformin’s ability to concomitantly target fibrosis and inflammation provides a plausible mechanism for its therapeutic effects in CCCA and other fibrosing alopecia disorders,” the authors concluded. But, they added, “larger prospective, placebo-controlled randomized clinical trials are needed to rigorously evaluate metformin’s efficacy and optimal dosing for treatment of cicatricial alopecias.”
SOURCE:
The study was led by Aaron Bao, Department of Dermatology, Johns Hopkins University School of Medicine, Baltimore, Maryland, and was published online on September 4 in JAMA Dermatology.
LIMITATIONS:
A small sample size, retrospective design, lack of a placebo control group, and the single-center setting limited the generalizability of the study findings. Additionally, the absence of a validated activity or severity scale for CCCA and the single posttreatment sampling limit the assessment and comparison of clinical symptoms and transcriptomic changes.
DISCLOSURES:
The study was supported by the American Academy of Dermatology. One author reported several ties with pharmaceutical companies, a pending patent, and authorship for the UpToDate section on CCCA.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Mortality Risk From Early-Onset CRC Higher in Rural, Poor Areas
TOPLINE:
Patients with early-onset colorectal cancer (CRC) living in rural and impoverished areas face a significantly higher risk of dying from CRC.
METHODOLOGY:
- Previous research has shown that patients living in impoverished and rural areas have an increased risk of dying from CRC, but it is unclear if this trend applies to patients with early-onset CRC.
- Researchers analyzed 58,200 patients with early-onset CRC from the Surveillance, Epidemiology, and End Results Program between 2006 and 2015.
- Of these patients, 1346 (21%) lived in rural areas with persistent poverty. Persistent poverty was defined as having 20% or more of the population living below the poverty level for about 30 years, and rural locations were identified using specific US Department of Agriculture codes.
- The primary outcome was cancer-specific survival.
TAKEAWAY:
- The cancer-specific survival at 5 years was highest for patients who lived in neither poverty-stricken nor rural areas (72%) and the lowest for those who lived in impoverished areas irrespective of rural status (67%).
- Patients who lived in rural areas had a significantly higher risk of dying from CRC than those living in nonrural areas, with younger individuals facing the highest risk. More specifically, patients aged between 20 and 29 years had a 35% higher risk of dying from CRC, those aged between 30 and 39 years had a 26% higher risk, and those aged between 40 and 49 years had a 12% higher risk.
- Patients who lived in poverty and rural areas had a 29% increased risk of dying from CRC compared with those in nonrural areas — with the highest 51% greater risk for those aged between 30 and 39 years.
IN PRACTICE:
“Our results can be used to inform health system policies for ongoing investments in cancer diagnosis and treatment resources in rural or impoverished areas for younger CRC patients and their communities,” the authors wrote.
SOURCE:
The study, led by Meng-Han Tsai, PhD, Georgia Prevention Institute, Augusta University, Augusta, Georgia, was published online in JAMA Network Open.
LIMITATIONS:
Confounders, such as lifestyle factors, comorbidities, and structural barriers, could affect the findings.
DISCLOSURES:
This study was partially supported by a grant from the Georgia Cancer Center Paceline funding mechanism at Augusta University. The authors did not declare any conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Patients with early-onset colorectal cancer (CRC) living in rural and impoverished areas face a significantly higher risk of dying from CRC.
METHODOLOGY:
- Previous research has shown that patients living in impoverished and rural areas have an increased risk of dying from CRC, but it is unclear if this trend applies to patients with early-onset CRC.
- Researchers analyzed 58,200 patients with early-onset CRC from the Surveillance, Epidemiology, and End Results Program between 2006 and 2015.
- Of these patients, 1346 (21%) lived in rural areas with persistent poverty. Persistent poverty was defined as having 20% or more of the population living below the poverty level for about 30 years, and rural locations were identified using specific US Department of Agriculture codes.
- The primary outcome was cancer-specific survival.
TAKEAWAY:
- The cancer-specific survival at 5 years was highest for patients who lived in neither poverty-stricken nor rural areas (72%) and the lowest for those who lived in impoverished areas irrespective of rural status (67%).
- Patients who lived in rural areas had a significantly higher risk of dying from CRC than those living in nonrural areas, with younger individuals facing the highest risk. More specifically, patients aged between 20 and 29 years had a 35% higher risk of dying from CRC, those aged between 30 and 39 years had a 26% higher risk, and those aged between 40 and 49 years had a 12% higher risk.
- Patients who lived in poverty and rural areas had a 29% increased risk of dying from CRC compared with those in nonrural areas — with the highest 51% greater risk for those aged between 30 and 39 years.
IN PRACTICE:
“Our results can be used to inform health system policies for ongoing investments in cancer diagnosis and treatment resources in rural or impoverished areas for younger CRC patients and their communities,” the authors wrote.
SOURCE:
The study, led by Meng-Han Tsai, PhD, Georgia Prevention Institute, Augusta University, Augusta, Georgia, was published online in JAMA Network Open.
LIMITATIONS:
Confounders, such as lifestyle factors, comorbidities, and structural barriers, could affect the findings.
DISCLOSURES:
This study was partially supported by a grant from the Georgia Cancer Center Paceline funding mechanism at Augusta University. The authors did not declare any conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Patients with early-onset colorectal cancer (CRC) living in rural and impoverished areas face a significantly higher risk of dying from CRC.
METHODOLOGY:
- Previous research has shown that patients living in impoverished and rural areas have an increased risk of dying from CRC, but it is unclear if this trend applies to patients with early-onset CRC.
- Researchers analyzed 58,200 patients with early-onset CRC from the Surveillance, Epidemiology, and End Results Program between 2006 and 2015.
- Of these patients, 1346 (21%) lived in rural areas with persistent poverty. Persistent poverty was defined as having 20% or more of the population living below the poverty level for about 30 years, and rural locations were identified using specific US Department of Agriculture codes.
- The primary outcome was cancer-specific survival.
TAKEAWAY:
- The cancer-specific survival at 5 years was highest for patients who lived in neither poverty-stricken nor rural areas (72%) and the lowest for those who lived in impoverished areas irrespective of rural status (67%).
- Patients who lived in rural areas had a significantly higher risk of dying from CRC than those living in nonrural areas, with younger individuals facing the highest risk. More specifically, patients aged between 20 and 29 years had a 35% higher risk of dying from CRC, those aged between 30 and 39 years had a 26% higher risk, and those aged between 40 and 49 years had a 12% higher risk.
- Patients who lived in poverty and rural areas had a 29% increased risk of dying from CRC compared with those in nonrural areas — with the highest 51% greater risk for those aged between 30 and 39 years.
IN PRACTICE:
“Our results can be used to inform health system policies for ongoing investments in cancer diagnosis and treatment resources in rural or impoverished areas for younger CRC patients and their communities,” the authors wrote.
SOURCE:
The study, led by Meng-Han Tsai, PhD, Georgia Prevention Institute, Augusta University, Augusta, Georgia, was published online in JAMA Network Open.
LIMITATIONS:
Confounders, such as lifestyle factors, comorbidities, and structural barriers, could affect the findings.
DISCLOSURES:
This study was partially supported by a grant from the Georgia Cancer Center Paceline funding mechanism at Augusta University. The authors did not declare any conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Enhanced Care for Pediatric Patients With Generalized Lichen Planus: Diagnosis and Treatment Tips
Practice Gap
Lichen planus (LP) is an inflammatory cutaneous disorder. Although it often is characterized by the 6 Ps—pruritic, polygonal, planar, purple, papules, and plaques with a predilection for the wrists and ankles—the presentation can vary in morphology and distribution.1-5 With an incidence of approximately 1% in the general population, LP is undoubtedly uncommon.1 Its prevalence in the pediatric population is especially low, with only 2% to 3% of cases manifesting in individuals younger than 20 years.2
Generalized LP (also referred to as eruptive or exanthematous LP) is a rarely reported clinical subtype in which lesions are disseminated or spread rapidly.5 The rarity of generalized LP in children often leads to misdiagnosis or delayed treatment, impacting the patient’s quality of life. Thus, there is a need for heightened awareness among clinicians on the variable presentation of LP in the pediatric population. Incorporating a punch biopsy for the diagnosis of LP when lesions manifest as widespread, erythematous to violaceous, flat-topped papules or plaques, along with the addition of an intramuscular (IM) injection in the treatment plan, improves overall patient outcomes.
Tools and Techniques
A detailed physical examination followed by a punch biopsy was critical for the diagnosis of generalized LP in a 7-year-old Black girl. The examination revealed a widespread distribution of dark, violaceous, polygonal, shiny, flat-topped, firm papules coalescing into plaques across the entire body, with a greater predilection for the legs and overlying joints (Figure, A). Some lesions exhibited fine, silver-white, reticular patterns consistent with Wickham striae. Notably, there was no involvement of the scalp, nails, or mucosal surfaces.
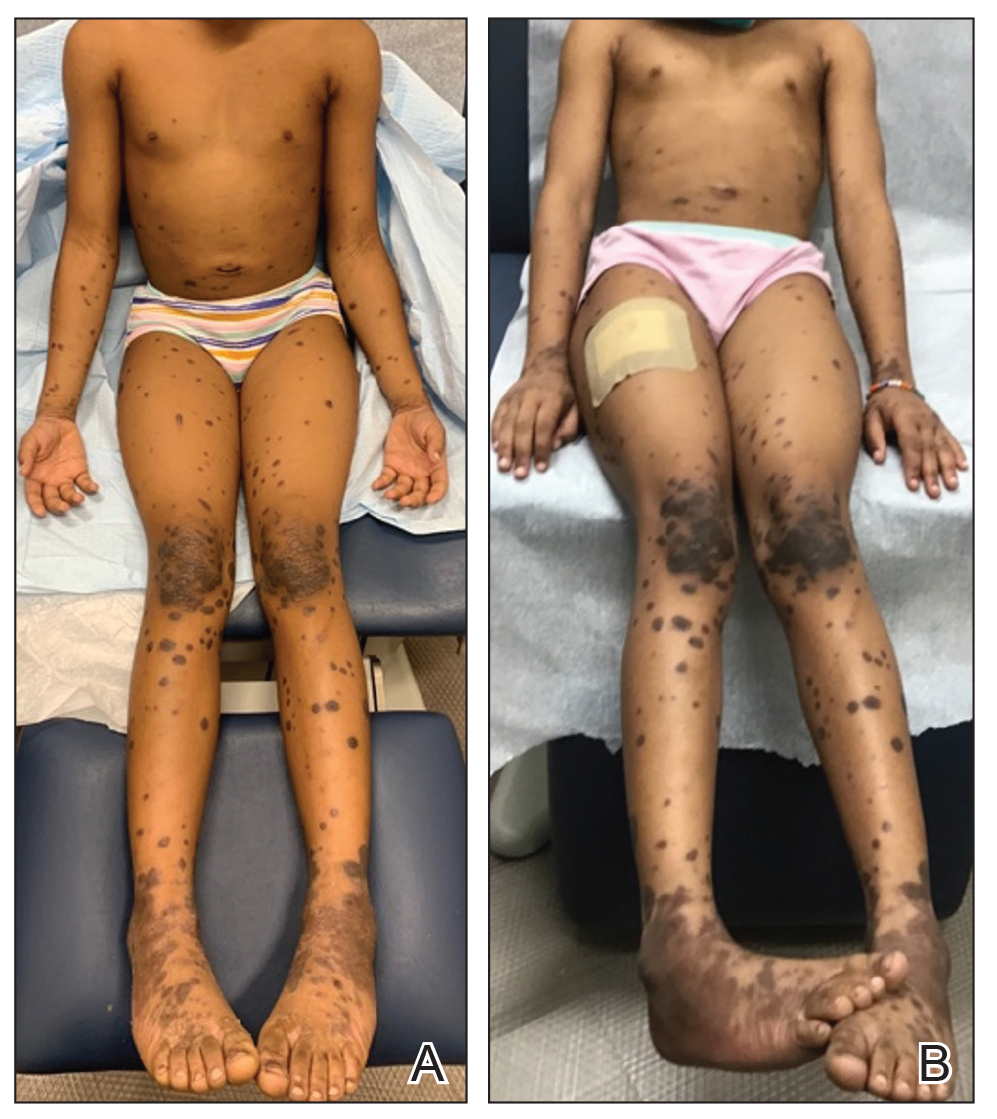
The patient had no relevant medical or family history of skin disease and no recent history of illness. She previously was treated by a pediatrician with triamcinolone cream 0.1%, a course of oral cephalexin, and oral cetirizine 10 mg once daily without relief of symptoms.
Although the clinical presentation was consistent with LP, the differential diagnosis included lichen simplex chronicus, atopic dermatitis, psoriasis, and generalized granuloma annulare. To address the need for early recognition of LP in pediatric patients, a punch biopsy of a lesion on the left anterior thigh was performed and showed lichenoid interface dermatitis—a pivotal finding in distinguishing LP from other conditions in the differential.
Given the patient’s age and severity of the LP, a combination of topical and systemic therapies was prescribed—clobetasol cream 0.025% twice daily and 1 injection of 0.5 cc of IM triamcinolone acetonide 40 mg/mL. This regimen was guided by the efficacy of IM injections in providing prompt symptomatic relief, particularly for patients with extensive disease or for those whose condition is refractory to topical treatments.6 Our patient achieved remarkable improvement at 2-week follow-up (Figure, B), without any observed adverse effects. At that time, the patient’s mother refused further systemic treatment and opted for only the topical therapy as well as natural light therapy.
Practice Implications
Timely and accurate diagnosis of LP in pediatric patients, especially those with skin of color, is crucial. Early intervention is especially important in mitigating the risk for chronic symptoms and preventing potential scarring, which tends to be more pronounced and challenging to treat in individuals with darker skin tones.7 Although not present in our patient, it is important to note that LP can affect the face (including the eyelids) as well as the palms and soles in pediatric patients with skin of color.
The most common approach to management of pediatric LP involves the use of a topical corticosteroid and an oral antihistamine, but the recalcitrant and generalized distribution of lesions warrants the administration of a systemic corticosteroid regardless of the patient’s age.6 In our patient, prompt administration of low-dose IM triamcinolone was both crucial and beneficial. Although an underutilized approach, IM triamcinolone helps to prevent the progression of lesions to the scalp, nails, and mucosa while also reducing inflammation and pruritus in glabrous skin.8
Triamcinolone acetonide injections—administered at concentrations of 5 to 40 mg/mL—directly into the lesion (0.5–1 cc per 2 cm2) are highly effective in managing recalcitrant thickened lesions such as those seen in hypertrophic LP and palmoplantar LP.6 This treatment is particularly beneficial when lesions are unresponsive to topical therapies. Administered every 3 to 6 weeks, these injections provide rapid symptom relief, typically within 72 hours,6 while also contributing to the reduction of lesion size and thickness over time. The concentration of triamcinolone acetonide should be selected based on the lesion’s severity, with higher concentrations reserved for thicker, more resistant lesions. More frequent injections may be warranted in cases in which rapid lesion reduction is necessary, while less frequent sessions may suffice for maintenance therapy. It is important to follow patients closely for adverse effects, such as signs of local skin atrophy or hypopigmentation, and to adjust the dose or frequency accordingly. To mitigate these risks, consider using the lowest effective concentration and rotating injection sites if treating multiple lesions. Additionally, combining intralesional corticosteroids with topical therapies can enhance outcomes, particularly in cases in which monotherapy is insufficient.
Patients should be monitored vigilantly for complications of LP. The risk for postinflammatory hyperpigmentation is a particular concern for patients with skin of color. Other complications of untreated LP include nail deformities and scarring alopecia.9 Regular and thorough follow-ups every few months to monitor scalp, mucosal, and genital involvement are essential to manage this risk effectively.
Furthermore, patient education is key. Informing patients and their caregivers about the nature of LP, the available treatment options, and the importance of ongoing follow-up can help to enhance treatment adherence and improve overall outcomes.
- Le Cleach L, Chosidow O. Clinical practice. Lichen planus. N Engl J Med. 2012;366:723-732. doi:10.1056/NEJMcp1103641
- Handa S, Sahoo B. Childhood lichen planus: a study of 87 cases. Int J Dermatol. 2002;41:423-427. doi:10.1046/j.1365-4362.2002.01522.x
- George J, Murray T, Bain M. Generalized, eruptive lichen planus in a pediatric patient. Contemp Pediatr. 2022;39:32-34.
- Arnold DL, Krishnamurthy K. Lichen planus. StatPearls [Internet]. Updated June 1, 2023. Accessed August 12, 2024. https://www.ncbi.nlm.nih.gov/books/NBK526126/
- Weston G, Payette M. Update on lichen planus and its clinical variants. Int J Womens Dermatol. 2015;1:140-149. doi:10.1016/j.ijwd.2015.04.001
- Mutalik SD, Belgaumkar VA, Rasal YD. Current perspectives in the treatment of childhood lichen planus. Indian J Paediatr Dermatol. 2021;22:316-325. doi:10.4103/ijpd.ijpd_165_20
- Usatine RP, Tinitigan M. Diagnosis and treatment of lichen planus. Am Fam Physician. 2011;84:53-60.
- Thomas LW, Elsensohn A, Bergheim T, et al. Intramuscular steroids in the treatment of dermatologic disease: a systematic review. J Drugs Dermatol. 2018;17:323-329.
- Gorouhi F, Davari P, Fazel N. Cutaneous and mucosal lichen planus: a comprehensive review of clinical subtypes, risk factors, diagnosis, and prognosis. ScientificWorldJournal. 2014;2014:742826. doi:10.1155/2014/742826
Practice Gap
Lichen planus (LP) is an inflammatory cutaneous disorder. Although it often is characterized by the 6 Ps—pruritic, polygonal, planar, purple, papules, and plaques with a predilection for the wrists and ankles—the presentation can vary in morphology and distribution.1-5 With an incidence of approximately 1% in the general population, LP is undoubtedly uncommon.1 Its prevalence in the pediatric population is especially low, with only 2% to 3% of cases manifesting in individuals younger than 20 years.2
Generalized LP (also referred to as eruptive or exanthematous LP) is a rarely reported clinical subtype in which lesions are disseminated or spread rapidly.5 The rarity of generalized LP in children often leads to misdiagnosis or delayed treatment, impacting the patient’s quality of life. Thus, there is a need for heightened awareness among clinicians on the variable presentation of LP in the pediatric population. Incorporating a punch biopsy for the diagnosis of LP when lesions manifest as widespread, erythematous to violaceous, flat-topped papules or plaques, along with the addition of an intramuscular (IM) injection in the treatment plan, improves overall patient outcomes.
Tools and Techniques
A detailed physical examination followed by a punch biopsy was critical for the diagnosis of generalized LP in a 7-year-old Black girl. The examination revealed a widespread distribution of dark, violaceous, polygonal, shiny, flat-topped, firm papules coalescing into plaques across the entire body, with a greater predilection for the legs and overlying joints (Figure, A). Some lesions exhibited fine, silver-white, reticular patterns consistent with Wickham striae. Notably, there was no involvement of the scalp, nails, or mucosal surfaces.

The patient had no relevant medical or family history of skin disease and no recent history of illness. She previously was treated by a pediatrician with triamcinolone cream 0.1%, a course of oral cephalexin, and oral cetirizine 10 mg once daily without relief of symptoms.
Although the clinical presentation was consistent with LP, the differential diagnosis included lichen simplex chronicus, atopic dermatitis, psoriasis, and generalized granuloma annulare. To address the need for early recognition of LP in pediatric patients, a punch biopsy of a lesion on the left anterior thigh was performed and showed lichenoid interface dermatitis—a pivotal finding in distinguishing LP from other conditions in the differential.
Given the patient’s age and severity of the LP, a combination of topical and systemic therapies was prescribed—clobetasol cream 0.025% twice daily and 1 injection of 0.5 cc of IM triamcinolone acetonide 40 mg/mL. This regimen was guided by the efficacy of IM injections in providing prompt symptomatic relief, particularly for patients with extensive disease or for those whose condition is refractory to topical treatments.6 Our patient achieved remarkable improvement at 2-week follow-up (Figure, B), without any observed adverse effects. At that time, the patient’s mother refused further systemic treatment and opted for only the topical therapy as well as natural light therapy.
Practice Implications
Timely and accurate diagnosis of LP in pediatric patients, especially those with skin of color, is crucial. Early intervention is especially important in mitigating the risk for chronic symptoms and preventing potential scarring, which tends to be more pronounced and challenging to treat in individuals with darker skin tones.7 Although not present in our patient, it is important to note that LP can affect the face (including the eyelids) as well as the palms and soles in pediatric patients with skin of color.
The most common approach to management of pediatric LP involves the use of a topical corticosteroid and an oral antihistamine, but the recalcitrant and generalized distribution of lesions warrants the administration of a systemic corticosteroid regardless of the patient’s age.6 In our patient, prompt administration of low-dose IM triamcinolone was both crucial and beneficial. Although an underutilized approach, IM triamcinolone helps to prevent the progression of lesions to the scalp, nails, and mucosa while also reducing inflammation and pruritus in glabrous skin.8
Triamcinolone acetonide injections—administered at concentrations of 5 to 40 mg/mL—directly into the lesion (0.5–1 cc per 2 cm2) are highly effective in managing recalcitrant thickened lesions such as those seen in hypertrophic LP and palmoplantar LP.6 This treatment is particularly beneficial when lesions are unresponsive to topical therapies. Administered every 3 to 6 weeks, these injections provide rapid symptom relief, typically within 72 hours,6 while also contributing to the reduction of lesion size and thickness over time. The concentration of triamcinolone acetonide should be selected based on the lesion’s severity, with higher concentrations reserved for thicker, more resistant lesions. More frequent injections may be warranted in cases in which rapid lesion reduction is necessary, while less frequent sessions may suffice for maintenance therapy. It is important to follow patients closely for adverse effects, such as signs of local skin atrophy or hypopigmentation, and to adjust the dose or frequency accordingly. To mitigate these risks, consider using the lowest effective concentration and rotating injection sites if treating multiple lesions. Additionally, combining intralesional corticosteroids with topical therapies can enhance outcomes, particularly in cases in which monotherapy is insufficient.
Patients should be monitored vigilantly for complications of LP. The risk for postinflammatory hyperpigmentation is a particular concern for patients with skin of color. Other complications of untreated LP include nail deformities and scarring alopecia.9 Regular and thorough follow-ups every few months to monitor scalp, mucosal, and genital involvement are essential to manage this risk effectively.
Furthermore, patient education is key. Informing patients and their caregivers about the nature of LP, the available treatment options, and the importance of ongoing follow-up can help to enhance treatment adherence and improve overall outcomes.
Practice Gap
Lichen planus (LP) is an inflammatory cutaneous disorder. Although it often is characterized by the 6 Ps—pruritic, polygonal, planar, purple, papules, and plaques with a predilection for the wrists and ankles—the presentation can vary in morphology and distribution.1-5 With an incidence of approximately 1% in the general population, LP is undoubtedly uncommon.1 Its prevalence in the pediatric population is especially low, with only 2% to 3% of cases manifesting in individuals younger than 20 years.2
Generalized LP (also referred to as eruptive or exanthematous LP) is a rarely reported clinical subtype in which lesions are disseminated or spread rapidly.5 The rarity of generalized LP in children often leads to misdiagnosis or delayed treatment, impacting the patient’s quality of life. Thus, there is a need for heightened awareness among clinicians on the variable presentation of LP in the pediatric population. Incorporating a punch biopsy for the diagnosis of LP when lesions manifest as widespread, erythematous to violaceous, flat-topped papules or plaques, along with the addition of an intramuscular (IM) injection in the treatment plan, improves overall patient outcomes.
Tools and Techniques
A detailed physical examination followed by a punch biopsy was critical for the diagnosis of generalized LP in a 7-year-old Black girl. The examination revealed a widespread distribution of dark, violaceous, polygonal, shiny, flat-topped, firm papules coalescing into plaques across the entire body, with a greater predilection for the legs and overlying joints (Figure, A). Some lesions exhibited fine, silver-white, reticular patterns consistent with Wickham striae. Notably, there was no involvement of the scalp, nails, or mucosal surfaces.

The patient had no relevant medical or family history of skin disease and no recent history of illness. She previously was treated by a pediatrician with triamcinolone cream 0.1%, a course of oral cephalexin, and oral cetirizine 10 mg once daily without relief of symptoms.
Although the clinical presentation was consistent with LP, the differential diagnosis included lichen simplex chronicus, atopic dermatitis, psoriasis, and generalized granuloma annulare. To address the need for early recognition of LP in pediatric patients, a punch biopsy of a lesion on the left anterior thigh was performed and showed lichenoid interface dermatitis—a pivotal finding in distinguishing LP from other conditions in the differential.
Given the patient’s age and severity of the LP, a combination of topical and systemic therapies was prescribed—clobetasol cream 0.025% twice daily and 1 injection of 0.5 cc of IM triamcinolone acetonide 40 mg/mL. This regimen was guided by the efficacy of IM injections in providing prompt symptomatic relief, particularly for patients with extensive disease or for those whose condition is refractory to topical treatments.6 Our patient achieved remarkable improvement at 2-week follow-up (Figure, B), without any observed adverse effects. At that time, the patient’s mother refused further systemic treatment and opted for only the topical therapy as well as natural light therapy.
Practice Implications
Timely and accurate diagnosis of LP in pediatric patients, especially those with skin of color, is crucial. Early intervention is especially important in mitigating the risk for chronic symptoms and preventing potential scarring, which tends to be more pronounced and challenging to treat in individuals with darker skin tones.7 Although not present in our patient, it is important to note that LP can affect the face (including the eyelids) as well as the palms and soles in pediatric patients with skin of color.
The most common approach to management of pediatric LP involves the use of a topical corticosteroid and an oral antihistamine, but the recalcitrant and generalized distribution of lesions warrants the administration of a systemic corticosteroid regardless of the patient’s age.6 In our patient, prompt administration of low-dose IM triamcinolone was both crucial and beneficial. Although an underutilized approach, IM triamcinolone helps to prevent the progression of lesions to the scalp, nails, and mucosa while also reducing inflammation and pruritus in glabrous skin.8
Triamcinolone acetonide injections—administered at concentrations of 5 to 40 mg/mL—directly into the lesion (0.5–1 cc per 2 cm2) are highly effective in managing recalcitrant thickened lesions such as those seen in hypertrophic LP and palmoplantar LP.6 This treatment is particularly beneficial when lesions are unresponsive to topical therapies. Administered every 3 to 6 weeks, these injections provide rapid symptom relief, typically within 72 hours,6 while also contributing to the reduction of lesion size and thickness over time. The concentration of triamcinolone acetonide should be selected based on the lesion’s severity, with higher concentrations reserved for thicker, more resistant lesions. More frequent injections may be warranted in cases in which rapid lesion reduction is necessary, while less frequent sessions may suffice for maintenance therapy. It is important to follow patients closely for adverse effects, such as signs of local skin atrophy or hypopigmentation, and to adjust the dose or frequency accordingly. To mitigate these risks, consider using the lowest effective concentration and rotating injection sites if treating multiple lesions. Additionally, combining intralesional corticosteroids with topical therapies can enhance outcomes, particularly in cases in which monotherapy is insufficient.
Patients should be monitored vigilantly for complications of LP. The risk for postinflammatory hyperpigmentation is a particular concern for patients with skin of color. Other complications of untreated LP include nail deformities and scarring alopecia.9 Regular and thorough follow-ups every few months to monitor scalp, mucosal, and genital involvement are essential to manage this risk effectively.
Furthermore, patient education is key. Informing patients and their caregivers about the nature of LP, the available treatment options, and the importance of ongoing follow-up can help to enhance treatment adherence and improve overall outcomes.
- Le Cleach L, Chosidow O. Clinical practice. Lichen planus. N Engl J Med. 2012;366:723-732. doi:10.1056/NEJMcp1103641
- Handa S, Sahoo B. Childhood lichen planus: a study of 87 cases. Int J Dermatol. 2002;41:423-427. doi:10.1046/j.1365-4362.2002.01522.x
- George J, Murray T, Bain M. Generalized, eruptive lichen planus in a pediatric patient. Contemp Pediatr. 2022;39:32-34.
- Arnold DL, Krishnamurthy K. Lichen planus. StatPearls [Internet]. Updated June 1, 2023. Accessed August 12, 2024. https://www.ncbi.nlm.nih.gov/books/NBK526126/
- Weston G, Payette M. Update on lichen planus and its clinical variants. Int J Womens Dermatol. 2015;1:140-149. doi:10.1016/j.ijwd.2015.04.001
- Mutalik SD, Belgaumkar VA, Rasal YD. Current perspectives in the treatment of childhood lichen planus. Indian J Paediatr Dermatol. 2021;22:316-325. doi:10.4103/ijpd.ijpd_165_20
- Usatine RP, Tinitigan M. Diagnosis and treatment of lichen planus. Am Fam Physician. 2011;84:53-60.
- Thomas LW, Elsensohn A, Bergheim T, et al. Intramuscular steroids in the treatment of dermatologic disease: a systematic review. J Drugs Dermatol. 2018;17:323-329.
- Gorouhi F, Davari P, Fazel N. Cutaneous and mucosal lichen planus: a comprehensive review of clinical subtypes, risk factors, diagnosis, and prognosis. ScientificWorldJournal. 2014;2014:742826. doi:10.1155/2014/742826
- Le Cleach L, Chosidow O. Clinical practice. Lichen planus. N Engl J Med. 2012;366:723-732. doi:10.1056/NEJMcp1103641
- Handa S, Sahoo B. Childhood lichen planus: a study of 87 cases. Int J Dermatol. 2002;41:423-427. doi:10.1046/j.1365-4362.2002.01522.x
- George J, Murray T, Bain M. Generalized, eruptive lichen planus in a pediatric patient. Contemp Pediatr. 2022;39:32-34.
- Arnold DL, Krishnamurthy K. Lichen planus. StatPearls [Internet]. Updated June 1, 2023. Accessed August 12, 2024. https://www.ncbi.nlm.nih.gov/books/NBK526126/
- Weston G, Payette M. Update on lichen planus and its clinical variants. Int J Womens Dermatol. 2015;1:140-149. doi:10.1016/j.ijwd.2015.04.001
- Mutalik SD, Belgaumkar VA, Rasal YD. Current perspectives in the treatment of childhood lichen planus. Indian J Paediatr Dermatol. 2021;22:316-325. doi:10.4103/ijpd.ijpd_165_20
- Usatine RP, Tinitigan M. Diagnosis and treatment of lichen planus. Am Fam Physician. 2011;84:53-60.
- Thomas LW, Elsensohn A, Bergheim T, et al. Intramuscular steroids in the treatment of dermatologic disease: a systematic review. J Drugs Dermatol. 2018;17:323-329.
- Gorouhi F, Davari P, Fazel N. Cutaneous and mucosal lichen planus: a comprehensive review of clinical subtypes, risk factors, diagnosis, and prognosis. ScientificWorldJournal. 2014;2014:742826. doi:10.1155/2014/742826