User login
Vast Majority of Adults At Risk for Cardiovascular-Kidney-Metabolic Syndrome
TOPLINE:
Nearly 90% of adults were at risk of developing cardiovascular-kidney-metabolic (CKM) syndrome between 2011 and 2020, according to new research published in JAMA.
METHODOLOGY:
- In 2023, the American Heart Association defined to acknowledge how heart and kidney diseases, diabetes, and obesity interact and are increasingly co-occurring conditions.
- Researchers used data from the National Health and Nutrition Examination Survey between 2011 and 2020.
- More than 10,000 adults over age 20 years were included; all of them received a physical and fasting laboratory measurements and self-reported their cardiovascular disease (CVD) status.
- Researchers created categories for risk, ranging from 0 (no risk factors) to 4, using factors such as kidney disease, obesity, and hypertension.
TAKEAWAY:
- (having metabolic risk factors like hypertension or moderate- to high-risk chronic kidney disease).
- 14.6% met the criteria for advanced stage 3 (very high-risk chronic kidney disease or a high risk for 10-year CVD) and stage 4 CKM syndrome (established CVD) combined.
- Men, adults over age 65 years, and Black individuals were at a greater risk for advanced stages of the CKM syndrome.
- Almost half of people met the criteria for stage 2 (having metabolic risk factors like hypertension or moderate- to high-risk chronic kidney disease).
- 14.6% met the criteria for advanced stage 3 (very high-risk chronic kidney disease or a high risk for 10-year CVD) and stage 4 CKM syndrome (established CVD) combined.
- Men, adults over age 65 years, and Black individuals were at a greater risk for advanced stages of the CKM syndrome.
IN PRACTICE:
“Equitable health care approaches prioritizing CKM health are urgently needed,” the study authors wrote.
SOURCE:
The study was led by Muthiah Vaduganathan, MD, MPH, cardiologist and researcher at Brigham and Women’s Hospital, Harvard Medical School, Boston.
LIMITATIONS:
Established CVD statuses were self-reported. Some data that would indicate advanced CKM stages were not available (eg, cardiac biomarkers, echocardiography, and coronary angiography), which may have led to an underestimation of rates.
DISCLOSURES:
One author received grants from Bristol Myers Squibb–Pfizer outside the submitted work. Dr. Vaduganathan received grants from and was an adviser and committee trial member for various pharmaceutical companies outside the submitted work. The authors reported no other disclosures.
A version of this article appeared on Medscape.com.
TOPLINE:
Nearly 90% of adults were at risk of developing cardiovascular-kidney-metabolic (CKM) syndrome between 2011 and 2020, according to new research published in JAMA.
METHODOLOGY:
- In 2023, the American Heart Association defined to acknowledge how heart and kidney diseases, diabetes, and obesity interact and are increasingly co-occurring conditions.
- Researchers used data from the National Health and Nutrition Examination Survey between 2011 and 2020.
- More than 10,000 adults over age 20 years were included; all of them received a physical and fasting laboratory measurements and self-reported their cardiovascular disease (CVD) status.
- Researchers created categories for risk, ranging from 0 (no risk factors) to 4, using factors such as kidney disease, obesity, and hypertension.
TAKEAWAY:
- (having metabolic risk factors like hypertension or moderate- to high-risk chronic kidney disease).
- 14.6% met the criteria for advanced stage 3 (very high-risk chronic kidney disease or a high risk for 10-year CVD) and stage 4 CKM syndrome (established CVD) combined.
- Men, adults over age 65 years, and Black individuals were at a greater risk for advanced stages of the CKM syndrome.
- Almost half of people met the criteria for stage 2 (having metabolic risk factors like hypertension or moderate- to high-risk chronic kidney disease).
- 14.6% met the criteria for advanced stage 3 (very high-risk chronic kidney disease or a high risk for 10-year CVD) and stage 4 CKM syndrome (established CVD) combined.
- Men, adults over age 65 years, and Black individuals were at a greater risk for advanced stages of the CKM syndrome.
IN PRACTICE:
“Equitable health care approaches prioritizing CKM health are urgently needed,” the study authors wrote.
SOURCE:
The study was led by Muthiah Vaduganathan, MD, MPH, cardiologist and researcher at Brigham and Women’s Hospital, Harvard Medical School, Boston.
LIMITATIONS:
Established CVD statuses were self-reported. Some data that would indicate advanced CKM stages were not available (eg, cardiac biomarkers, echocardiography, and coronary angiography), which may have led to an underestimation of rates.
DISCLOSURES:
One author received grants from Bristol Myers Squibb–Pfizer outside the submitted work. Dr. Vaduganathan received grants from and was an adviser and committee trial member for various pharmaceutical companies outside the submitted work. The authors reported no other disclosures.
A version of this article appeared on Medscape.com.
TOPLINE:
Nearly 90% of adults were at risk of developing cardiovascular-kidney-metabolic (CKM) syndrome between 2011 and 2020, according to new research published in JAMA.
METHODOLOGY:
- In 2023, the American Heart Association defined to acknowledge how heart and kidney diseases, diabetes, and obesity interact and are increasingly co-occurring conditions.
- Researchers used data from the National Health and Nutrition Examination Survey between 2011 and 2020.
- More than 10,000 adults over age 20 years were included; all of them received a physical and fasting laboratory measurements and self-reported their cardiovascular disease (CVD) status.
- Researchers created categories for risk, ranging from 0 (no risk factors) to 4, using factors such as kidney disease, obesity, and hypertension.
TAKEAWAY:
- (having metabolic risk factors like hypertension or moderate- to high-risk chronic kidney disease).
- 14.6% met the criteria for advanced stage 3 (very high-risk chronic kidney disease or a high risk for 10-year CVD) and stage 4 CKM syndrome (established CVD) combined.
- Men, adults over age 65 years, and Black individuals were at a greater risk for advanced stages of the CKM syndrome.
- Almost half of people met the criteria for stage 2 (having metabolic risk factors like hypertension or moderate- to high-risk chronic kidney disease).
- 14.6% met the criteria for advanced stage 3 (very high-risk chronic kidney disease or a high risk for 10-year CVD) and stage 4 CKM syndrome (established CVD) combined.
- Men, adults over age 65 years, and Black individuals were at a greater risk for advanced stages of the CKM syndrome.
IN PRACTICE:
“Equitable health care approaches prioritizing CKM health are urgently needed,” the study authors wrote.
SOURCE:
The study was led by Muthiah Vaduganathan, MD, MPH, cardiologist and researcher at Brigham and Women’s Hospital, Harvard Medical School, Boston.
LIMITATIONS:
Established CVD statuses were self-reported. Some data that would indicate advanced CKM stages were not available (eg, cardiac biomarkers, echocardiography, and coronary angiography), which may have led to an underestimation of rates.
DISCLOSURES:
One author received grants from Bristol Myers Squibb–Pfizer outside the submitted work. Dr. Vaduganathan received grants from and was an adviser and committee trial member for various pharmaceutical companies outside the submitted work. The authors reported no other disclosures.
A version of this article appeared on Medscape.com.
For Pediatric LGS, Cenobamate Shows Promise
DENVER — The conclusions were reached by comparing outcomes to patient historical data, though they were not analyzed statistically.
A Proof-of-Concept Study
In an interview, Karen Keough, MD, who presented the study during a poster session at the 2024 annual meeting of the American Academy of Neurology, conceded the key limitation was that the researchers were not able to perform statistical analysis due to the nature of the data. “It’s just showing trends. It’s proof of concept that cenobamate can be effective in one of the most refractory forms of epilepsy, which always includes focal seizures. That’s what led to the initial FDA indication, but we also know that this medication has a lot of promise and is probably going to be effective in other forms of epilepsy and probably in other seizure types,” said Dr. Keough, who is a neurologist at Pediatrix Child Neurology Consultants of Austin, Texas.
Although she has seen significant improvements in many patients, Dr. Keough reported that most patients become refractory again. “Unfortunately, honeymoons are probably real in cenobamate. Continuing to follow those patients as I do, since they’re my own patients, I have quite a few who had more than a year of seizure freedom, [but] their seizures are back, though not as bad as they were before cenobamate. I just can’t get them back to that 100% control category. I do have a few that are still in the 100% control category, but not very many,” she said.
She also presented a retrospective chart review of 36 LGS patients between the ages of 1 and 27 years at the American Epilepsy Society in December 2023, which showed that addition of cenobamate was associated with a reduction in seizure frequency in 85% of patients. “It was a profound number of patients who had long periods of seizure freedom,” she said.
A Promising Treatment Option
Dr. Keough is considering moving cenobamate up in the treatment sequence of new LGS patients. “I don’t use cenobamate first line in anyone because we have good first-line agents for simple epilepsy in Lennox-Gastaut, but I’m bringing out cenobamate pretty early in the course, because I do think it has superior efficacy compared with most other drugs that we have available. Most of my patients are very established and they’ve seen lots of other drugs. For many patients, there are only a couple of drugs left on the list that [they] have never tried, but we’re going to put cenobamate at the top of that. For my newer diagnoses, I’m going to bring it out much earlier,” she said, though other drugs such as clobazam (Onfi) would still rank ahead of cenobamate.
The study was drawn from records of the HealthVerity Marketplace Database, which includes more than 150 commercial, Medicare, and Medicaid payers. It included 76 patients aged 17 or under who took at least one antiseizure medication between May 2020 and December 2022, and who had filled at least two prescriptions of cenobamate and had 180 or more days of medical and pharmacy enrollment. The mean age was 13.4 years (5.3% 0-5 years, 15.8% 6-11 years, 78.9% 12-17 years), and 40.8% were female. Seizure types included absence (17.1%), focal (75.0%), and generalized tonic-clonic (86.8%). All patients had a history of intractable seizures, 80.3% had a history of status epilepticus, and 28.9% had a history of infantile seizures. A little more than one fourth (27.6%) of patients had commercial insurance. In the previous 90 days, 21.1% had had an emergency room visit or in-patient hospital stay.
Common antiseizure medications taken with cenobamate included cannabidiol (n = 14), clobazam (n = 8), and levetiracetam (n = 18).
During the cenobamate treatment period, patients had a lower incidence of epilepsy-related inpatient days per year (3.36 vs 3.94), epilepsy-related ER visits per year (0.66 vs 1.19), and likelihood of requiring a new line of epilepsy therapy (35.5% vs 100%).
‘Promising’ Results, but More Research Is Needed
The fact that cenobamate was used in combination with other therapies, plus the lack of a control group, makes it difficult to determine if cenobamate was actually responsible for the improvements, according to Nassim Zecavati, MD, who was asked for comment on the study. “I think the results are promising, but there’s obviously a need for a randomized, controlled trial to understand whether it was this medication or the combination of cenobamate with [other medications]. How do we know that this isn’t a compound effect, that it’s multifactorial, and a combination of multiple medications versus the cenobamate?” she said.
Still, she noted that LGS patients are highly vulnerable to hospital admissions and status epilepticus, making the parameters examined in the study valid and important. “I think that this drug likely has a role in the treatment of patients with LGS, particularly pediatric patients. I think we just need more data,” said Dr. Zecavati, who is director of Epilepsy at the Children’s Hospital of Richmond in Virginia and associate professor of Neurology at Virginia Commonwealth University.
Dr. Keough is a speaker for SK Life Sciences. Dr. Zecavati has no relevant financial disclosures.
DENVER — The conclusions were reached by comparing outcomes to patient historical data, though they were not analyzed statistically.
A Proof-of-Concept Study
In an interview, Karen Keough, MD, who presented the study during a poster session at the 2024 annual meeting of the American Academy of Neurology, conceded the key limitation was that the researchers were not able to perform statistical analysis due to the nature of the data. “It’s just showing trends. It’s proof of concept that cenobamate can be effective in one of the most refractory forms of epilepsy, which always includes focal seizures. That’s what led to the initial FDA indication, but we also know that this medication has a lot of promise and is probably going to be effective in other forms of epilepsy and probably in other seizure types,” said Dr. Keough, who is a neurologist at Pediatrix Child Neurology Consultants of Austin, Texas.
Although she has seen significant improvements in many patients, Dr. Keough reported that most patients become refractory again. “Unfortunately, honeymoons are probably real in cenobamate. Continuing to follow those patients as I do, since they’re my own patients, I have quite a few who had more than a year of seizure freedom, [but] their seizures are back, though not as bad as they were before cenobamate. I just can’t get them back to that 100% control category. I do have a few that are still in the 100% control category, but not very many,” she said.
She also presented a retrospective chart review of 36 LGS patients between the ages of 1 and 27 years at the American Epilepsy Society in December 2023, which showed that addition of cenobamate was associated with a reduction in seizure frequency in 85% of patients. “It was a profound number of patients who had long periods of seizure freedom,” she said.
A Promising Treatment Option
Dr. Keough is considering moving cenobamate up in the treatment sequence of new LGS patients. “I don’t use cenobamate first line in anyone because we have good first-line agents for simple epilepsy in Lennox-Gastaut, but I’m bringing out cenobamate pretty early in the course, because I do think it has superior efficacy compared with most other drugs that we have available. Most of my patients are very established and they’ve seen lots of other drugs. For many patients, there are only a couple of drugs left on the list that [they] have never tried, but we’re going to put cenobamate at the top of that. For my newer diagnoses, I’m going to bring it out much earlier,” she said, though other drugs such as clobazam (Onfi) would still rank ahead of cenobamate.
The study was drawn from records of the HealthVerity Marketplace Database, which includes more than 150 commercial, Medicare, and Medicaid payers. It included 76 patients aged 17 or under who took at least one antiseizure medication between May 2020 and December 2022, and who had filled at least two prescriptions of cenobamate and had 180 or more days of medical and pharmacy enrollment. The mean age was 13.4 years (5.3% 0-5 years, 15.8% 6-11 years, 78.9% 12-17 years), and 40.8% were female. Seizure types included absence (17.1%), focal (75.0%), and generalized tonic-clonic (86.8%). All patients had a history of intractable seizures, 80.3% had a history of status epilepticus, and 28.9% had a history of infantile seizures. A little more than one fourth (27.6%) of patients had commercial insurance. In the previous 90 days, 21.1% had had an emergency room visit or in-patient hospital stay.
Common antiseizure medications taken with cenobamate included cannabidiol (n = 14), clobazam (n = 8), and levetiracetam (n = 18).
During the cenobamate treatment period, patients had a lower incidence of epilepsy-related inpatient days per year (3.36 vs 3.94), epilepsy-related ER visits per year (0.66 vs 1.19), and likelihood of requiring a new line of epilepsy therapy (35.5% vs 100%).
‘Promising’ Results, but More Research Is Needed
The fact that cenobamate was used in combination with other therapies, plus the lack of a control group, makes it difficult to determine if cenobamate was actually responsible for the improvements, according to Nassim Zecavati, MD, who was asked for comment on the study. “I think the results are promising, but there’s obviously a need for a randomized, controlled trial to understand whether it was this medication or the combination of cenobamate with [other medications]. How do we know that this isn’t a compound effect, that it’s multifactorial, and a combination of multiple medications versus the cenobamate?” she said.
Still, she noted that LGS patients are highly vulnerable to hospital admissions and status epilepticus, making the parameters examined in the study valid and important. “I think that this drug likely has a role in the treatment of patients with LGS, particularly pediatric patients. I think we just need more data,” said Dr. Zecavati, who is director of Epilepsy at the Children’s Hospital of Richmond in Virginia and associate professor of Neurology at Virginia Commonwealth University.
Dr. Keough is a speaker for SK Life Sciences. Dr. Zecavati has no relevant financial disclosures.
DENVER — The conclusions were reached by comparing outcomes to patient historical data, though they were not analyzed statistically.
A Proof-of-Concept Study
In an interview, Karen Keough, MD, who presented the study during a poster session at the 2024 annual meeting of the American Academy of Neurology, conceded the key limitation was that the researchers were not able to perform statistical analysis due to the nature of the data. “It’s just showing trends. It’s proof of concept that cenobamate can be effective in one of the most refractory forms of epilepsy, which always includes focal seizures. That’s what led to the initial FDA indication, but we also know that this medication has a lot of promise and is probably going to be effective in other forms of epilepsy and probably in other seizure types,” said Dr. Keough, who is a neurologist at Pediatrix Child Neurology Consultants of Austin, Texas.
Although she has seen significant improvements in many patients, Dr. Keough reported that most patients become refractory again. “Unfortunately, honeymoons are probably real in cenobamate. Continuing to follow those patients as I do, since they’re my own patients, I have quite a few who had more than a year of seizure freedom, [but] their seizures are back, though not as bad as they were before cenobamate. I just can’t get them back to that 100% control category. I do have a few that are still in the 100% control category, but not very many,” she said.
She also presented a retrospective chart review of 36 LGS patients between the ages of 1 and 27 years at the American Epilepsy Society in December 2023, which showed that addition of cenobamate was associated with a reduction in seizure frequency in 85% of patients. “It was a profound number of patients who had long periods of seizure freedom,” she said.
A Promising Treatment Option
Dr. Keough is considering moving cenobamate up in the treatment sequence of new LGS patients. “I don’t use cenobamate first line in anyone because we have good first-line agents for simple epilepsy in Lennox-Gastaut, but I’m bringing out cenobamate pretty early in the course, because I do think it has superior efficacy compared with most other drugs that we have available. Most of my patients are very established and they’ve seen lots of other drugs. For many patients, there are only a couple of drugs left on the list that [they] have never tried, but we’re going to put cenobamate at the top of that. For my newer diagnoses, I’m going to bring it out much earlier,” she said, though other drugs such as clobazam (Onfi) would still rank ahead of cenobamate.
The study was drawn from records of the HealthVerity Marketplace Database, which includes more than 150 commercial, Medicare, and Medicaid payers. It included 76 patients aged 17 or under who took at least one antiseizure medication between May 2020 and December 2022, and who had filled at least two prescriptions of cenobamate and had 180 or more days of medical and pharmacy enrollment. The mean age was 13.4 years (5.3% 0-5 years, 15.8% 6-11 years, 78.9% 12-17 years), and 40.8% were female. Seizure types included absence (17.1%), focal (75.0%), and generalized tonic-clonic (86.8%). All patients had a history of intractable seizures, 80.3% had a history of status epilepticus, and 28.9% had a history of infantile seizures. A little more than one fourth (27.6%) of patients had commercial insurance. In the previous 90 days, 21.1% had had an emergency room visit or in-patient hospital stay.
Common antiseizure medications taken with cenobamate included cannabidiol (n = 14), clobazam (n = 8), and levetiracetam (n = 18).
During the cenobamate treatment period, patients had a lower incidence of epilepsy-related inpatient days per year (3.36 vs 3.94), epilepsy-related ER visits per year (0.66 vs 1.19), and likelihood of requiring a new line of epilepsy therapy (35.5% vs 100%).
‘Promising’ Results, but More Research Is Needed
The fact that cenobamate was used in combination with other therapies, plus the lack of a control group, makes it difficult to determine if cenobamate was actually responsible for the improvements, according to Nassim Zecavati, MD, who was asked for comment on the study. “I think the results are promising, but there’s obviously a need for a randomized, controlled trial to understand whether it was this medication or the combination of cenobamate with [other medications]. How do we know that this isn’t a compound effect, that it’s multifactorial, and a combination of multiple medications versus the cenobamate?” she said.
Still, she noted that LGS patients are highly vulnerable to hospital admissions and status epilepticus, making the parameters examined in the study valid and important. “I think that this drug likely has a role in the treatment of patients with LGS, particularly pediatric patients. I think we just need more data,” said Dr. Zecavati, who is director of Epilepsy at the Children’s Hospital of Richmond in Virginia and associate professor of Neurology at Virginia Commonwealth University.
Dr. Keough is a speaker for SK Life Sciences. Dr. Zecavati has no relevant financial disclosures.
FROM AAN 2024
Purpuric Eruption in a Patient With Hairy Cell Leukemia
The Diagnosis: Purpuric Drug Eruption
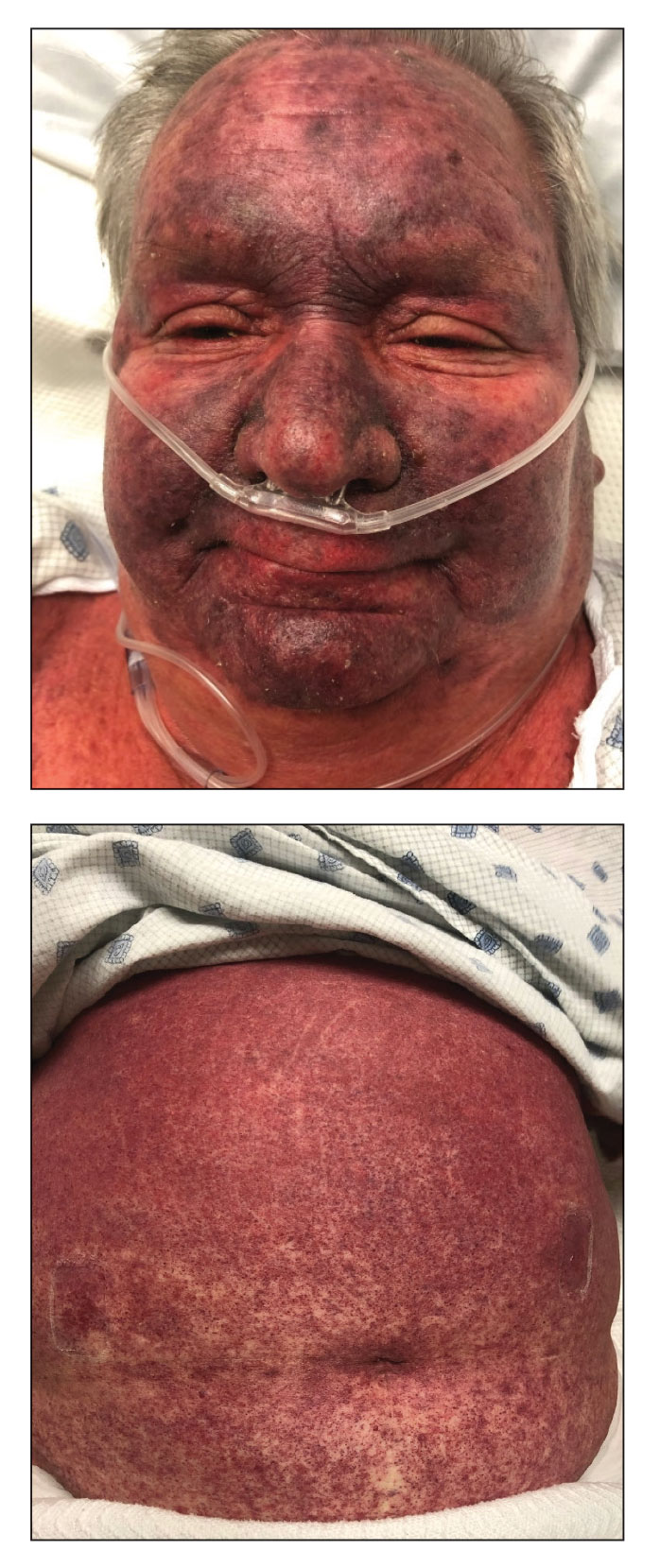
Histopathology revealed interface dermatitis, spongiosis, and a perivascular lymphocytic infiltrate with extravasated red blood cells consistent with a purpuric drug eruption. Our patient achieved remission of hairy cell leukemia after receiving only 2 of 5 expected doses of cladribine. The rash resolved completely in 3 weeks following a prednisone taper (Figure).
Hairy cell leukemia is a rare indolent lymphoproliferative disorder of B cells that accounts for approximately 2% of adult leukemias in the United States. Cladribine, a purine nucleoside analog that impairs DNA synthesis and repair, has become the mainstay of therapy, demonstrating a 95% complete response rate.1 Although few reports have addressed the cutaneous reactions seen with cladribine therapy, they can occur in more than 50% of patients.1,2 The most common skin manifestation associated with cladribine therapy is a morbilliform rash, but Stevens-Johnson syndrome and toxic epidermal necrolysis (TEN) have been reported.1
Few cases of purpuric eruption secondary to cladribine treatment have been described, and nearly all reports involve concomitant medications such as allopurinol, which our patient was taking, and antibiotics including trimethoprim-sulfamethoxazole and penicillins.1,3,4 In a cohort of 35 patients receiving cladribine,1 only concomitant treatment with cladribine and allopurinol caused cutaneous reactions, further supporting the hypothesis of cladribine-induced drug sensitivity. Allopurinol often is prescribed during induction therapy for prophylaxis against tumor lysis syndrome; similarly, antibiotics frequently are given prophylactically and therapeutically for neutropenic fever. It is believed that T-cell imbalance and profound lymphopenia induced by cladribine increase susceptibility to drug hypersensitivity reactions.1,3
The typical purpuric eruption develops within 2 days of starting cladribine therapy. Diascopy will reveal petechiae, and biopsy should be performed to rule out other serious drug-induced reactions, such as erythema multiforme, Stevens-Johnson syndrome, and TEN. A cladribine-induced purpuric eruption typically is self-resolving and carries a favorable prognosis, though high-dose corticosteroids often are prescribed to hasten recovery. The rare reports of serious cutaneous reactions secondary to cladribine therapy have been with maculopapular, not purpuric eruptions.2 Based on limited available data, cladribine-induced purpura should not be a limitation to continued treatment in patients who need it.1 Careful consideration of concomitant drug use is necessary, as the current literature demonstrates resolution of rash with withdrawal of other therapies, namely allopurinol.2-4 Future studies are needed to examine the safety of withholding offending medications and to further elucidate the mechanisms contributing to drug hypersensitivity due to cladribine.
Widespread purpura and petechiae can pose a wide differential; the patient’s recent history of cladribine administration pointed to a classic purpuric eruption. Other diagnoses such as toxic erythema of chemotherapy (TEC) and TEN are not purpuric, though plaques can be violaceous. Lack of bullae, blisters, and facial or mucosal surface involvement suggest TEN.5 Thrombotic thrombocytopenic purpura and disseminated intravascular coagulation do manifest with petechiae and purpura, though such a robust eruption in the context of recent cladribine therapy is less likely. The classic retiform purpura and necrosis were not present to suggest purpura fulminans from disseminated intravascular coagulation.
Several of the proposed diagnoses as well as a purpuric drug eruption would demonstrate extravasated red blood cells on histopathology, but the presence of interface dermatitis narrows the differential to a purpuric drug eruption. Necrotic keratinocytes and full-thickness necrosis were not present on biopsy to support a diagnosis of TEN in our patient. Characteristic features of TEC—including eccrine squamous syringometaplasia, dermal edema, and keratinocyte atypia—were not present on biopsy.6 Finally, although TEN should resolve with steroid treatment, TEC is self-limited and thrombotic thrombocytopenic purpura and disseminated intravascular coagulation would not resolve with use of steroids alone.
- Ganzel C, Gatt ME, Maly A, et al. High incidence of skin rash in patients with hairy cell leukemia treated with cladribine. Leuk Lymphoma. 2012;53:1169-1173. doi:10.3109/10428194.2011.635864
- Chubar Y, Bennett M. Cutaneous reactions in hairy cell leukaemia treated with 2-chlorodeoxyadenosine and allopurinol. Br J Haematol. 2003;122:768-770. doi:10.1046/j.1365-2141.2003.04506.x
- Espinosa Lara P, Quirós Redondo V, Aguado Lobo M, et al. Purpuric exanthema in a patient with hairy cell leukemia treated with cladribine and allopurinol. Ann Hematol. 2017;96:1209-1210. doi:10.1007 /s00277-017-2992-z
- Hendrick A. Purpuric rash following treatment with 2-chlorodeoxyadenosine. Clin Lab Haematol. 2001;23:67-68. doi:10.1046 /j.1365-2257.2001.0346b.x
- Kang S, Amagai M, Bruckner AL, et al, eds. Fitzpatrick’s Dermatology. 9th ed. McGraw-Hill Education; 2019.
- Bolognia JL, Cooper DL, Glusac EJ. Toxic erythema of chemotherapy: a useful clinical term. J Am Acad Dermatol. 2008;59:524-529.
The Diagnosis: Purpuric Drug Eruption
Histopathology revealed interface dermatitis, spongiosis, and a perivascular lymphocytic infiltrate with extravasated red blood cells consistent with a purpuric drug eruption. Our patient achieved remission of hairy cell leukemia after receiving only 2 of 5 expected doses of cladribine. The rash resolved completely in 3 weeks following a prednisone taper (Figure).

Hairy cell leukemia is a rare indolent lymphoproliferative disorder of B cells that accounts for approximately 2% of adult leukemias in the United States. Cladribine, a purine nucleoside analog that impairs DNA synthesis and repair, has become the mainstay of therapy, demonstrating a 95% complete response rate.1 Although few reports have addressed the cutaneous reactions seen with cladribine therapy, they can occur in more than 50% of patients.1,2 The most common skin manifestation associated with cladribine therapy is a morbilliform rash, but Stevens-Johnson syndrome and toxic epidermal necrolysis (TEN) have been reported.1
Few cases of purpuric eruption secondary to cladribine treatment have been described, and nearly all reports involve concomitant medications such as allopurinol, which our patient was taking, and antibiotics including trimethoprim-sulfamethoxazole and penicillins.1,3,4 In a cohort of 35 patients receiving cladribine,1 only concomitant treatment with cladribine and allopurinol caused cutaneous reactions, further supporting the hypothesis of cladribine-induced drug sensitivity. Allopurinol often is prescribed during induction therapy for prophylaxis against tumor lysis syndrome; similarly, antibiotics frequently are given prophylactically and therapeutically for neutropenic fever. It is believed that T-cell imbalance and profound lymphopenia induced by cladribine increase susceptibility to drug hypersensitivity reactions.1,3
The typical purpuric eruption develops within 2 days of starting cladribine therapy. Diascopy will reveal petechiae, and biopsy should be performed to rule out other serious drug-induced reactions, such as erythema multiforme, Stevens-Johnson syndrome, and TEN. A cladribine-induced purpuric eruption typically is self-resolving and carries a favorable prognosis, though high-dose corticosteroids often are prescribed to hasten recovery. The rare reports of serious cutaneous reactions secondary to cladribine therapy have been with maculopapular, not purpuric eruptions.2 Based on limited available data, cladribine-induced purpura should not be a limitation to continued treatment in patients who need it.1 Careful consideration of concomitant drug use is necessary, as the current literature demonstrates resolution of rash with withdrawal of other therapies, namely allopurinol.2-4 Future studies are needed to examine the safety of withholding offending medications and to further elucidate the mechanisms contributing to drug hypersensitivity due to cladribine.
Widespread purpura and petechiae can pose a wide differential; the patient’s recent history of cladribine administration pointed to a classic purpuric eruption. Other diagnoses such as toxic erythema of chemotherapy (TEC) and TEN are not purpuric, though plaques can be violaceous. Lack of bullae, blisters, and facial or mucosal surface involvement suggest TEN.5 Thrombotic thrombocytopenic purpura and disseminated intravascular coagulation do manifest with petechiae and purpura, though such a robust eruption in the context of recent cladribine therapy is less likely. The classic retiform purpura and necrosis were not present to suggest purpura fulminans from disseminated intravascular coagulation.
Several of the proposed diagnoses as well as a purpuric drug eruption would demonstrate extravasated red blood cells on histopathology, but the presence of interface dermatitis narrows the differential to a purpuric drug eruption. Necrotic keratinocytes and full-thickness necrosis were not present on biopsy to support a diagnosis of TEN in our patient. Characteristic features of TEC—including eccrine squamous syringometaplasia, dermal edema, and keratinocyte atypia—were not present on biopsy.6 Finally, although TEN should resolve with steroid treatment, TEC is self-limited and thrombotic thrombocytopenic purpura and disseminated intravascular coagulation would not resolve with use of steroids alone.
The Diagnosis: Purpuric Drug Eruption
Histopathology revealed interface dermatitis, spongiosis, and a perivascular lymphocytic infiltrate with extravasated red blood cells consistent with a purpuric drug eruption. Our patient achieved remission of hairy cell leukemia after receiving only 2 of 5 expected doses of cladribine. The rash resolved completely in 3 weeks following a prednisone taper (Figure).

Hairy cell leukemia is a rare indolent lymphoproliferative disorder of B cells that accounts for approximately 2% of adult leukemias in the United States. Cladribine, a purine nucleoside analog that impairs DNA synthesis and repair, has become the mainstay of therapy, demonstrating a 95% complete response rate.1 Although few reports have addressed the cutaneous reactions seen with cladribine therapy, they can occur in more than 50% of patients.1,2 The most common skin manifestation associated with cladribine therapy is a morbilliform rash, but Stevens-Johnson syndrome and toxic epidermal necrolysis (TEN) have been reported.1
Few cases of purpuric eruption secondary to cladribine treatment have been described, and nearly all reports involve concomitant medications such as allopurinol, which our patient was taking, and antibiotics including trimethoprim-sulfamethoxazole and penicillins.1,3,4 In a cohort of 35 patients receiving cladribine,1 only concomitant treatment with cladribine and allopurinol caused cutaneous reactions, further supporting the hypothesis of cladribine-induced drug sensitivity. Allopurinol often is prescribed during induction therapy for prophylaxis against tumor lysis syndrome; similarly, antibiotics frequently are given prophylactically and therapeutically for neutropenic fever. It is believed that T-cell imbalance and profound lymphopenia induced by cladribine increase susceptibility to drug hypersensitivity reactions.1,3
The typical purpuric eruption develops within 2 days of starting cladribine therapy. Diascopy will reveal petechiae, and biopsy should be performed to rule out other serious drug-induced reactions, such as erythema multiforme, Stevens-Johnson syndrome, and TEN. A cladribine-induced purpuric eruption typically is self-resolving and carries a favorable prognosis, though high-dose corticosteroids often are prescribed to hasten recovery. The rare reports of serious cutaneous reactions secondary to cladribine therapy have been with maculopapular, not purpuric eruptions.2 Based on limited available data, cladribine-induced purpura should not be a limitation to continued treatment in patients who need it.1 Careful consideration of concomitant drug use is necessary, as the current literature demonstrates resolution of rash with withdrawal of other therapies, namely allopurinol.2-4 Future studies are needed to examine the safety of withholding offending medications and to further elucidate the mechanisms contributing to drug hypersensitivity due to cladribine.
Widespread purpura and petechiae can pose a wide differential; the patient’s recent history of cladribine administration pointed to a classic purpuric eruption. Other diagnoses such as toxic erythema of chemotherapy (TEC) and TEN are not purpuric, though plaques can be violaceous. Lack of bullae, blisters, and facial or mucosal surface involvement suggest TEN.5 Thrombotic thrombocytopenic purpura and disseminated intravascular coagulation do manifest with petechiae and purpura, though such a robust eruption in the context of recent cladribine therapy is less likely. The classic retiform purpura and necrosis were not present to suggest purpura fulminans from disseminated intravascular coagulation.
Several of the proposed diagnoses as well as a purpuric drug eruption would demonstrate extravasated red blood cells on histopathology, but the presence of interface dermatitis narrows the differential to a purpuric drug eruption. Necrotic keratinocytes and full-thickness necrosis were not present on biopsy to support a diagnosis of TEN in our patient. Characteristic features of TEC—including eccrine squamous syringometaplasia, dermal edema, and keratinocyte atypia—were not present on biopsy.6 Finally, although TEN should resolve with steroid treatment, TEC is self-limited and thrombotic thrombocytopenic purpura and disseminated intravascular coagulation would not resolve with use of steroids alone.
- Ganzel C, Gatt ME, Maly A, et al. High incidence of skin rash in patients with hairy cell leukemia treated with cladribine. Leuk Lymphoma. 2012;53:1169-1173. doi:10.3109/10428194.2011.635864
- Chubar Y, Bennett M. Cutaneous reactions in hairy cell leukaemia treated with 2-chlorodeoxyadenosine and allopurinol. Br J Haematol. 2003;122:768-770. doi:10.1046/j.1365-2141.2003.04506.x
- Espinosa Lara P, Quirós Redondo V, Aguado Lobo M, et al. Purpuric exanthema in a patient with hairy cell leukemia treated with cladribine and allopurinol. Ann Hematol. 2017;96:1209-1210. doi:10.1007 /s00277-017-2992-z
- Hendrick A. Purpuric rash following treatment with 2-chlorodeoxyadenosine. Clin Lab Haematol. 2001;23:67-68. doi:10.1046 /j.1365-2257.2001.0346b.x
- Kang S, Amagai M, Bruckner AL, et al, eds. Fitzpatrick’s Dermatology. 9th ed. McGraw-Hill Education; 2019.
- Bolognia JL, Cooper DL, Glusac EJ. Toxic erythema of chemotherapy: a useful clinical term. J Am Acad Dermatol. 2008;59:524-529.
- Ganzel C, Gatt ME, Maly A, et al. High incidence of skin rash in patients with hairy cell leukemia treated with cladribine. Leuk Lymphoma. 2012;53:1169-1173. doi:10.3109/10428194.2011.635864
- Chubar Y, Bennett M. Cutaneous reactions in hairy cell leukaemia treated with 2-chlorodeoxyadenosine and allopurinol. Br J Haematol. 2003;122:768-770. doi:10.1046/j.1365-2141.2003.04506.x
- Espinosa Lara P, Quirós Redondo V, Aguado Lobo M, et al. Purpuric exanthema in a patient with hairy cell leukemia treated with cladribine and allopurinol. Ann Hematol. 2017;96:1209-1210. doi:10.1007 /s00277-017-2992-z
- Hendrick A. Purpuric rash following treatment with 2-chlorodeoxyadenosine. Clin Lab Haematol. 2001;23:67-68. doi:10.1046 /j.1365-2257.2001.0346b.x
- Kang S, Amagai M, Bruckner AL, et al, eds. Fitzpatrick’s Dermatology. 9th ed. McGraw-Hill Education; 2019.
- Bolognia JL, Cooper DL, Glusac EJ. Toxic erythema of chemotherapy: a useful clinical term. J Am Acad Dermatol. 2008;59:524-529.
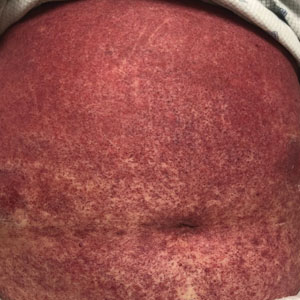
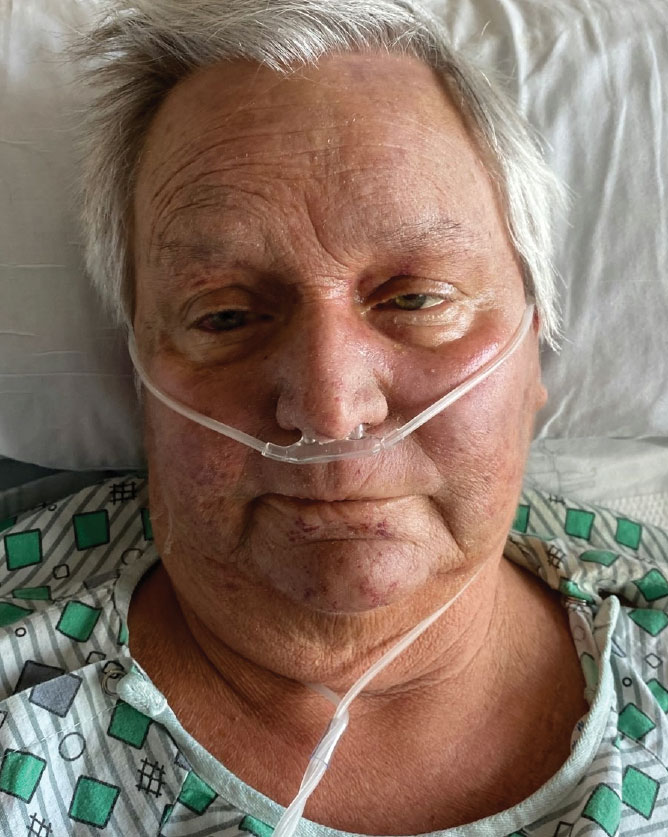
A 68-year-old woman presented to the emergency department with neutropenic fever and a rash over the body after receiving 2 doses of cladribine therapy for hairy cell leukemia. Physical examination demonstrated marked facial (top), lip, and tongue swelling, as well as a diffuse dusky nonpalpable purpuric rash on the abdomen (bottom) and back involving 90% of the body surface area. Bilateral ear edema was appreciated with accentuation of the earlobe crease. The patient exhibited subconjunctival hemorrhage, ectropion, and scleral injection. A punch biopsy of the thigh was performed.
Plastic Surgeon Illegally Restricted Negative Reviews, Judge Rules
A plastic surgeon broke federal law when he restricted patients from posting negative reviews by requiring them to sign nondisclosure agreements before they received care, a district judge has ruled.
Seattle-based surgeon Javad Sajan, MD, ran afoul of the Consumer Review Fairness Act (CRFA) by requiring more than 10,000 patients to sign the agreements, according to a recent decision by US District Judge Ricardo S. Martinez. The law protects consumers’ rights to post truthful reviews about businesses.
Judge Martinez wrote that the terms of Dr. Sajan’s nondisclosure agreements “clearly include language prohibiting or restricting patients from posting negative reviews,” in violation of CRFA. Penalties for the offense will be determined at a September trial.
This news organization contacted Dr. Sajan’s office and his attorney for comment but did not get a response.
The decision is the latest development in an ongoing legal dispute between Dr. Sajan and the State of Washington over whether the surgeon’s efforts to limit negative online reviews were illegal.
Beginning in 2017, Dr. Sajan and his practice, Allure Esthetic, introduced agreements that “forced” patients to contact the business directly if they had concerns rather than post a negative review, according to a 2022 lawsuit against Dr. Sajan filed by Washington Attorney General Robert Ferguson.
“Online reviews are often the first stop when consumers are determining who to trust,” Mr. Ferguson said in a statement. “That’s especially critical when those services deal with a patient’s health and safety. We will take action against those who illegally stop Washingtonians from sharing reviews with the public.”
If patients posted negative reviews, the clinic, in some cases, threatened litigation, according to the complaint. In other cases, patients were allegedly offered money and free services in exchange for taking the reviews down. Patients who accepted cash or services were required to sign a second agreement forbidding them from posting future negative reviews and imposing a $250,000 penalty for failure to comply, according to court documents.
In court documents, Dr. Sajan’s attorneys argued the agreements did not violate CRFA because patients had the opportunity to modify the language or decline signing them, which hundreds did. The CRFA requires Mr. Ferguson to prove that consumers lacked a meaningful opportunity to negotiate the terms, attorneys for Dr. Sajan argued in court records.
But Judge Martinez wrote that the patients who declined to sign the agreements or changed the terms represented only a “tiny fraction” of the affected patients.
The agreement language restricts patients from speaking out by forcing dissatisfied patients to work with Allure until a resolution is reached, Judge Martinez noted in his decision. “At the very least, this would delay patients from posting such reviews and force patients to interact in some way with Allure, and it certainly appears to prohibit posting reviews until Allure agrees to some kind of favorable resolution.”
Surgeon Posted Fake Positive Reviews to Counteract Bad Reviews, AG Says
Employee accounts in court documents describe a physician fixated on reviews who went to great lengths to ensure positive reviews about his work outweighed the negative.
Former employees said they were instructed to track down patients who left negative reviews and either “threaten” them to take the posts down or offer them “money” or other things, according to Mr. Ferguson’s lawsuit. If patients could not be identified, the practice would file a defamation lawsuit against the anonymous person who posted the review and use litigation to subpoena the website for the reviewer’s IP address in order to identify them, according to court documents.
Employees testified they had regular meetings to review current negative reviews and discuss what steps they were taking to get them removed. At team meetings, in-house counsel would regularly present an Excel spreadsheet with updates on progress in getting patients to remove negative reviews, according to court documents.
In addition to restricting negative reviews, Mr. Ferguson accuses Dr. Sajan of posting fake positive reviews and “buying” thousands of fake followers on social media.
At Dr. Sajan’s direction, employees created Gmail accounts using stock photos for their profile pictures and used the accounts to post fake reviews of Allure Esthetic and Dr. Sajan, according to the complaint. The practice also used members of an online forum called BlackHatWorld.com to create fake email accounts and to post fake reviews, the attorney general alleges. Many of the fake positive reviews, including the fake Google reviews, still appear on online review sites today, the attorney general contends.
Dr. Sajan and his practice also allegedly manipulated social media to appear more popular. Mr. Ferguson claims that Dr. Sajan instructed his former web designer to purchase 60,000 followers through a vendor on BlackHatWorld.com. Most of Dr. Sajan’s current Instagram followers are not real, according to Mr. Ferguson.
The practice also used a social media bot tool to buy thousands of fake likes on Instagram, YouTube, and other social media, according to court documents.
In addition, Dr. Sajan and his practice are accused of significantly altering “before and after” photos of patients and using fake email accounts to allow the clinic to take skincare rebates intended for patients.
All of these practices violated HIPAA, the state Consumer Protection Act (CPA) and the federal CRFA, according to Mr. Ferguson.
Surgeon Claims Competitor Behind Allegations
Attorneys for Dr. Sajan argue a competitor is behind the accusations and that other regulatory entities determined the practice did nothing wrong.
The competitor, a Seattle-based plastic surgeon, filed numerous complaints about Dr. Sajan to the Washington Medical Commission (WMC), according to court documents. The medical commission reviewed the third agreement and closed its investigation, finding that if the allegations were true, “no violation of law occurred,” court records show.
“Defendants relied upon this closing code from the WMC that the (non-disclosure) forms were lawful,” Dr. Sajan’s attorneys wrote in court documents.
The US Department of Health & Human Services Office for Civil Rights (OCR) also reviewed and audited Dr. Sajan’s use of the agreements, his attorneys noted. In a notice from OCR included in court exhibits, the agency wrote that all matters at issue have now been resolved through the practice’s voluntary compliance actions and that it was closing its investigation.
Attorneys for Dr. Sajan accuse Mr. Ferguson and state investigators of withholding the full extent of the competitor’s involvement in their investigation and failing to identify the competitor in written discovery or any of its initial disclosures. Dr. Sajan and his team discovered that the competitor was a source of key information through public records requests, according to court documents.
The remaining claims against Dr. Sajan will be addressed at trial, set for September 9, 2024.
A version of this article appeared on Medscape.com.
A plastic surgeon broke federal law when he restricted patients from posting negative reviews by requiring them to sign nondisclosure agreements before they received care, a district judge has ruled.
Seattle-based surgeon Javad Sajan, MD, ran afoul of the Consumer Review Fairness Act (CRFA) by requiring more than 10,000 patients to sign the agreements, according to a recent decision by US District Judge Ricardo S. Martinez. The law protects consumers’ rights to post truthful reviews about businesses.
Judge Martinez wrote that the terms of Dr. Sajan’s nondisclosure agreements “clearly include language prohibiting or restricting patients from posting negative reviews,” in violation of CRFA. Penalties for the offense will be determined at a September trial.
This news organization contacted Dr. Sajan’s office and his attorney for comment but did not get a response.
The decision is the latest development in an ongoing legal dispute between Dr. Sajan and the State of Washington over whether the surgeon’s efforts to limit negative online reviews were illegal.
Beginning in 2017, Dr. Sajan and his practice, Allure Esthetic, introduced agreements that “forced” patients to contact the business directly if they had concerns rather than post a negative review, according to a 2022 lawsuit against Dr. Sajan filed by Washington Attorney General Robert Ferguson.
“Online reviews are often the first stop when consumers are determining who to trust,” Mr. Ferguson said in a statement. “That’s especially critical when those services deal with a patient’s health and safety. We will take action against those who illegally stop Washingtonians from sharing reviews with the public.”
If patients posted negative reviews, the clinic, in some cases, threatened litigation, according to the complaint. In other cases, patients were allegedly offered money and free services in exchange for taking the reviews down. Patients who accepted cash or services were required to sign a second agreement forbidding them from posting future negative reviews and imposing a $250,000 penalty for failure to comply, according to court documents.
In court documents, Dr. Sajan’s attorneys argued the agreements did not violate CRFA because patients had the opportunity to modify the language or decline signing them, which hundreds did. The CRFA requires Mr. Ferguson to prove that consumers lacked a meaningful opportunity to negotiate the terms, attorneys for Dr. Sajan argued in court records.
But Judge Martinez wrote that the patients who declined to sign the agreements or changed the terms represented only a “tiny fraction” of the affected patients.
The agreement language restricts patients from speaking out by forcing dissatisfied patients to work with Allure until a resolution is reached, Judge Martinez noted in his decision. “At the very least, this would delay patients from posting such reviews and force patients to interact in some way with Allure, and it certainly appears to prohibit posting reviews until Allure agrees to some kind of favorable resolution.”
Surgeon Posted Fake Positive Reviews to Counteract Bad Reviews, AG Says
Employee accounts in court documents describe a physician fixated on reviews who went to great lengths to ensure positive reviews about his work outweighed the negative.
Former employees said they were instructed to track down patients who left negative reviews and either “threaten” them to take the posts down or offer them “money” or other things, according to Mr. Ferguson’s lawsuit. If patients could not be identified, the practice would file a defamation lawsuit against the anonymous person who posted the review and use litigation to subpoena the website for the reviewer’s IP address in order to identify them, according to court documents.
Employees testified they had regular meetings to review current negative reviews and discuss what steps they were taking to get them removed. At team meetings, in-house counsel would regularly present an Excel spreadsheet with updates on progress in getting patients to remove negative reviews, according to court documents.
In addition to restricting negative reviews, Mr. Ferguson accuses Dr. Sajan of posting fake positive reviews and “buying” thousands of fake followers on social media.
At Dr. Sajan’s direction, employees created Gmail accounts using stock photos for their profile pictures and used the accounts to post fake reviews of Allure Esthetic and Dr. Sajan, according to the complaint. The practice also used members of an online forum called BlackHatWorld.com to create fake email accounts and to post fake reviews, the attorney general alleges. Many of the fake positive reviews, including the fake Google reviews, still appear on online review sites today, the attorney general contends.
Dr. Sajan and his practice also allegedly manipulated social media to appear more popular. Mr. Ferguson claims that Dr. Sajan instructed his former web designer to purchase 60,000 followers through a vendor on BlackHatWorld.com. Most of Dr. Sajan’s current Instagram followers are not real, according to Mr. Ferguson.
The practice also used a social media bot tool to buy thousands of fake likes on Instagram, YouTube, and other social media, according to court documents.
In addition, Dr. Sajan and his practice are accused of significantly altering “before and after” photos of patients and using fake email accounts to allow the clinic to take skincare rebates intended for patients.
All of these practices violated HIPAA, the state Consumer Protection Act (CPA) and the federal CRFA, according to Mr. Ferguson.
Surgeon Claims Competitor Behind Allegations
Attorneys for Dr. Sajan argue a competitor is behind the accusations and that other regulatory entities determined the practice did nothing wrong.
The competitor, a Seattle-based plastic surgeon, filed numerous complaints about Dr. Sajan to the Washington Medical Commission (WMC), according to court documents. The medical commission reviewed the third agreement and closed its investigation, finding that if the allegations were true, “no violation of law occurred,” court records show.
“Defendants relied upon this closing code from the WMC that the (non-disclosure) forms were lawful,” Dr. Sajan’s attorneys wrote in court documents.
The US Department of Health & Human Services Office for Civil Rights (OCR) also reviewed and audited Dr. Sajan’s use of the agreements, his attorneys noted. In a notice from OCR included in court exhibits, the agency wrote that all matters at issue have now been resolved through the practice’s voluntary compliance actions and that it was closing its investigation.
Attorneys for Dr. Sajan accuse Mr. Ferguson and state investigators of withholding the full extent of the competitor’s involvement in their investigation and failing to identify the competitor in written discovery or any of its initial disclosures. Dr. Sajan and his team discovered that the competitor was a source of key information through public records requests, according to court documents.
The remaining claims against Dr. Sajan will be addressed at trial, set for September 9, 2024.
A version of this article appeared on Medscape.com.
A plastic surgeon broke federal law when he restricted patients from posting negative reviews by requiring them to sign nondisclosure agreements before they received care, a district judge has ruled.
Seattle-based surgeon Javad Sajan, MD, ran afoul of the Consumer Review Fairness Act (CRFA) by requiring more than 10,000 patients to sign the agreements, according to a recent decision by US District Judge Ricardo S. Martinez. The law protects consumers’ rights to post truthful reviews about businesses.
Judge Martinez wrote that the terms of Dr. Sajan’s nondisclosure agreements “clearly include language prohibiting or restricting patients from posting negative reviews,” in violation of CRFA. Penalties for the offense will be determined at a September trial.
This news organization contacted Dr. Sajan’s office and his attorney for comment but did not get a response.
The decision is the latest development in an ongoing legal dispute between Dr. Sajan and the State of Washington over whether the surgeon’s efforts to limit negative online reviews were illegal.
Beginning in 2017, Dr. Sajan and his practice, Allure Esthetic, introduced agreements that “forced” patients to contact the business directly if they had concerns rather than post a negative review, according to a 2022 lawsuit against Dr. Sajan filed by Washington Attorney General Robert Ferguson.
“Online reviews are often the first stop when consumers are determining who to trust,” Mr. Ferguson said in a statement. “That’s especially critical when those services deal with a patient’s health and safety. We will take action against those who illegally stop Washingtonians from sharing reviews with the public.”
If patients posted negative reviews, the clinic, in some cases, threatened litigation, according to the complaint. In other cases, patients were allegedly offered money and free services in exchange for taking the reviews down. Patients who accepted cash or services were required to sign a second agreement forbidding them from posting future negative reviews and imposing a $250,000 penalty for failure to comply, according to court documents.
In court documents, Dr. Sajan’s attorneys argued the agreements did not violate CRFA because patients had the opportunity to modify the language or decline signing them, which hundreds did. The CRFA requires Mr. Ferguson to prove that consumers lacked a meaningful opportunity to negotiate the terms, attorneys for Dr. Sajan argued in court records.
But Judge Martinez wrote that the patients who declined to sign the agreements or changed the terms represented only a “tiny fraction” of the affected patients.
The agreement language restricts patients from speaking out by forcing dissatisfied patients to work with Allure until a resolution is reached, Judge Martinez noted in his decision. “At the very least, this would delay patients from posting such reviews and force patients to interact in some way with Allure, and it certainly appears to prohibit posting reviews until Allure agrees to some kind of favorable resolution.”
Surgeon Posted Fake Positive Reviews to Counteract Bad Reviews, AG Says
Employee accounts in court documents describe a physician fixated on reviews who went to great lengths to ensure positive reviews about his work outweighed the negative.
Former employees said they were instructed to track down patients who left negative reviews and either “threaten” them to take the posts down or offer them “money” or other things, according to Mr. Ferguson’s lawsuit. If patients could not be identified, the practice would file a defamation lawsuit against the anonymous person who posted the review and use litigation to subpoena the website for the reviewer’s IP address in order to identify them, according to court documents.
Employees testified they had regular meetings to review current negative reviews and discuss what steps they were taking to get them removed. At team meetings, in-house counsel would regularly present an Excel spreadsheet with updates on progress in getting patients to remove negative reviews, according to court documents.
In addition to restricting negative reviews, Mr. Ferguson accuses Dr. Sajan of posting fake positive reviews and “buying” thousands of fake followers on social media.
At Dr. Sajan’s direction, employees created Gmail accounts using stock photos for their profile pictures and used the accounts to post fake reviews of Allure Esthetic and Dr. Sajan, according to the complaint. The practice also used members of an online forum called BlackHatWorld.com to create fake email accounts and to post fake reviews, the attorney general alleges. Many of the fake positive reviews, including the fake Google reviews, still appear on online review sites today, the attorney general contends.
Dr. Sajan and his practice also allegedly manipulated social media to appear more popular. Mr. Ferguson claims that Dr. Sajan instructed his former web designer to purchase 60,000 followers through a vendor on BlackHatWorld.com. Most of Dr. Sajan’s current Instagram followers are not real, according to Mr. Ferguson.
The practice also used a social media bot tool to buy thousands of fake likes on Instagram, YouTube, and other social media, according to court documents.
In addition, Dr. Sajan and his practice are accused of significantly altering “before and after” photos of patients and using fake email accounts to allow the clinic to take skincare rebates intended for patients.
All of these practices violated HIPAA, the state Consumer Protection Act (CPA) and the federal CRFA, according to Mr. Ferguson.
Surgeon Claims Competitor Behind Allegations
Attorneys for Dr. Sajan argue a competitor is behind the accusations and that other regulatory entities determined the practice did nothing wrong.
The competitor, a Seattle-based plastic surgeon, filed numerous complaints about Dr. Sajan to the Washington Medical Commission (WMC), according to court documents. The medical commission reviewed the third agreement and closed its investigation, finding that if the allegations were true, “no violation of law occurred,” court records show.
“Defendants relied upon this closing code from the WMC that the (non-disclosure) forms were lawful,” Dr. Sajan’s attorneys wrote in court documents.
The US Department of Health & Human Services Office for Civil Rights (OCR) also reviewed and audited Dr. Sajan’s use of the agreements, his attorneys noted. In a notice from OCR included in court exhibits, the agency wrote that all matters at issue have now been resolved through the practice’s voluntary compliance actions and that it was closing its investigation.
Attorneys for Dr. Sajan accuse Mr. Ferguson and state investigators of withholding the full extent of the competitor’s involvement in their investigation and failing to identify the competitor in written discovery or any of its initial disclosures. Dr. Sajan and his team discovered that the competitor was a source of key information through public records requests, according to court documents.
The remaining claims against Dr. Sajan will be addressed at trial, set for September 9, 2024.
A version of this article appeared on Medscape.com.
Composite Scale Better Gauges Mucosal Injury in Celiac Disease
, according to a study in Clinical Gastroenterology and Hepatology.
The new morphometric duodenal biopsy mucosal scale joins together villous height-to-crypt depth ratio (Vh:Cd) and intraepithelial lymphocytes (IEL) — each key CeD histological measures of the small intestine — in a scale called VCIEL.
The authors believe the VCIEL will enable a broader and more accurate measurement of mucosal health in CeD. It will be particularly useful for population analysis in clinical trials and could improve the powering of trial design. “Use of VCIEL may lead to better outcome measures for potential new therapeutic treatments benefiting patients,” wrote Jocelyn A. Silvester, MD, PhD, a pediatrician at Boston Children’s Hospital and an assistant professor at Harvard Medical School, and colleagues.
This chronic enteropathy affects about 1% of the world’s population and requires a lifelong adherence to a gluten-free diet, the authors noted.
The authors pointed to weaknesses in the current quantitative and qualitative ways of measuring gluten-induced mucosal injury on biopsy for CeD. “Morphometry measures the injury continuum for architecture and inflammation, but these are used as separate outcomes,” they wrote. “The original Marsh-Oberhuber [M-O] classifications are rather contrived approaches to assess a biologic continuum, forcing the injury in categorical groups of unclear clinical relevance and where clinically significant changes may occur within one single category.”
Moreover, the quantitation of inflammation relies on binary assessment as normal or increased, which results in histology that is unscorable by M-O if villous atrophy persists without increased IELs, they added.
The Study
In the absence of a broadly accepted single measure of mucosal injury in CeD, the group assessed whether the composite metric could improve statistical precision for assessing histology.
Enter VCIEL, which combines the Vh:Cd and IEL for individual patients with equal weighting by converting each scale to a fraction of their standard deviation and summing the results.
The researchers applied the VCIEL formula in a reanalysis of four clinical gluten-challenge trials and compared the results for Vh:Cd and IEL separately with those for VCIEL for clinical significance (effect size) and statistical significance.
In reanalysis of the ALV003-1021 trial, for example, the researchers observed an effect size and P value (analysis of covariance) of 1.37 and .038 for a delta (difference) value of Vh:Cd 1.17 and .005 for IEL and 1.86 and .004 for VCIEL.
For the similar gluten-challenge IMGX003-NCCIH-1721 trial, the corresponding delta results were .76 and .057 for Vh:Cd, .98 and .018 for IEL, and 1.14 and .007 for VCIEL. Comparable improvements with VCIEL over individual Vh:Cd and IEL were observed for other studies, including a nontherapeutic gluten challenge study.
In NCT03409796 trial data, the computation of VCIEL values showed an improved statistical significance relative to the component values of Vh:Cd and IEL by the within-group paired 2-tailed t test P values from baseline to day 15, particularly at a 10-g gluten challenge dose: Vh:Cd, IEL, VCIEL = .0050, .0031, and .0014, respectively.
Little correlation emerged between baseline values and changes with intervention for Vh:Cd and IEL on an individual patient basis.
The greater accuracy and statistical precision of the VCIEL scale are presumably due to averaging over some of the measurement uncertainty in individual patient and timepoint Vh:Cd and IEL values and creating a composite of different histologic properties, the authors noted.
This study was funded by ImmunogenX, Inc. First author Jack A. Syage is a cofounder and shareholder in ImmunogenX Inc. Dr. Silvester has served on an advisory board for Takeda Pharmaceuticals and has received research funding from Biomedal S.L., Cour Pharmaceuticals, and Glutenostics LLC. Several coauthors disclosed various financial ties to multiple private-sector pharmaceutical and biomedical companies, including ImmunogenX.
, according to a study in Clinical Gastroenterology and Hepatology.
The new morphometric duodenal biopsy mucosal scale joins together villous height-to-crypt depth ratio (Vh:Cd) and intraepithelial lymphocytes (IEL) — each key CeD histological measures of the small intestine — in a scale called VCIEL.
The authors believe the VCIEL will enable a broader and more accurate measurement of mucosal health in CeD. It will be particularly useful for population analysis in clinical trials and could improve the powering of trial design. “Use of VCIEL may lead to better outcome measures for potential new therapeutic treatments benefiting patients,” wrote Jocelyn A. Silvester, MD, PhD, a pediatrician at Boston Children’s Hospital and an assistant professor at Harvard Medical School, and colleagues.
This chronic enteropathy affects about 1% of the world’s population and requires a lifelong adherence to a gluten-free diet, the authors noted.
The authors pointed to weaknesses in the current quantitative and qualitative ways of measuring gluten-induced mucosal injury on biopsy for CeD. “Morphometry measures the injury continuum for architecture and inflammation, but these are used as separate outcomes,” they wrote. “The original Marsh-Oberhuber [M-O] classifications are rather contrived approaches to assess a biologic continuum, forcing the injury in categorical groups of unclear clinical relevance and where clinically significant changes may occur within one single category.”
Moreover, the quantitation of inflammation relies on binary assessment as normal or increased, which results in histology that is unscorable by M-O if villous atrophy persists without increased IELs, they added.
The Study
In the absence of a broadly accepted single measure of mucosal injury in CeD, the group assessed whether the composite metric could improve statistical precision for assessing histology.
Enter VCIEL, which combines the Vh:Cd and IEL for individual patients with equal weighting by converting each scale to a fraction of their standard deviation and summing the results.
The researchers applied the VCIEL formula in a reanalysis of four clinical gluten-challenge trials and compared the results for Vh:Cd and IEL separately with those for VCIEL for clinical significance (effect size) and statistical significance.
In reanalysis of the ALV003-1021 trial, for example, the researchers observed an effect size and P value (analysis of covariance) of 1.37 and .038 for a delta (difference) value of Vh:Cd 1.17 and .005 for IEL and 1.86 and .004 for VCIEL.
For the similar gluten-challenge IMGX003-NCCIH-1721 trial, the corresponding delta results were .76 and .057 for Vh:Cd, .98 and .018 for IEL, and 1.14 and .007 for VCIEL. Comparable improvements with VCIEL over individual Vh:Cd and IEL were observed for other studies, including a nontherapeutic gluten challenge study.
In NCT03409796 trial data, the computation of VCIEL values showed an improved statistical significance relative to the component values of Vh:Cd and IEL by the within-group paired 2-tailed t test P values from baseline to day 15, particularly at a 10-g gluten challenge dose: Vh:Cd, IEL, VCIEL = .0050, .0031, and .0014, respectively.
Little correlation emerged between baseline values and changes with intervention for Vh:Cd and IEL on an individual patient basis.
The greater accuracy and statistical precision of the VCIEL scale are presumably due to averaging over some of the measurement uncertainty in individual patient and timepoint Vh:Cd and IEL values and creating a composite of different histologic properties, the authors noted.
This study was funded by ImmunogenX, Inc. First author Jack A. Syage is a cofounder and shareholder in ImmunogenX Inc. Dr. Silvester has served on an advisory board for Takeda Pharmaceuticals and has received research funding from Biomedal S.L., Cour Pharmaceuticals, and Glutenostics LLC. Several coauthors disclosed various financial ties to multiple private-sector pharmaceutical and biomedical companies, including ImmunogenX.
, according to a study in Clinical Gastroenterology and Hepatology.
The new morphometric duodenal biopsy mucosal scale joins together villous height-to-crypt depth ratio (Vh:Cd) and intraepithelial lymphocytes (IEL) — each key CeD histological measures of the small intestine — in a scale called VCIEL.
The authors believe the VCIEL will enable a broader and more accurate measurement of mucosal health in CeD. It will be particularly useful for population analysis in clinical trials and could improve the powering of trial design. “Use of VCIEL may lead to better outcome measures for potential new therapeutic treatments benefiting patients,” wrote Jocelyn A. Silvester, MD, PhD, a pediatrician at Boston Children’s Hospital and an assistant professor at Harvard Medical School, and colleagues.
This chronic enteropathy affects about 1% of the world’s population and requires a lifelong adherence to a gluten-free diet, the authors noted.
The authors pointed to weaknesses in the current quantitative and qualitative ways of measuring gluten-induced mucosal injury on biopsy for CeD. “Morphometry measures the injury continuum for architecture and inflammation, but these are used as separate outcomes,” they wrote. “The original Marsh-Oberhuber [M-O] classifications are rather contrived approaches to assess a biologic continuum, forcing the injury in categorical groups of unclear clinical relevance and where clinically significant changes may occur within one single category.”
Moreover, the quantitation of inflammation relies on binary assessment as normal or increased, which results in histology that is unscorable by M-O if villous atrophy persists without increased IELs, they added.
The Study
In the absence of a broadly accepted single measure of mucosal injury in CeD, the group assessed whether the composite metric could improve statistical precision for assessing histology.
Enter VCIEL, which combines the Vh:Cd and IEL for individual patients with equal weighting by converting each scale to a fraction of their standard deviation and summing the results.
The researchers applied the VCIEL formula in a reanalysis of four clinical gluten-challenge trials and compared the results for Vh:Cd and IEL separately with those for VCIEL for clinical significance (effect size) and statistical significance.
In reanalysis of the ALV003-1021 trial, for example, the researchers observed an effect size and P value (analysis of covariance) of 1.37 and .038 for a delta (difference) value of Vh:Cd 1.17 and .005 for IEL and 1.86 and .004 for VCIEL.
For the similar gluten-challenge IMGX003-NCCIH-1721 trial, the corresponding delta results were .76 and .057 for Vh:Cd, .98 and .018 for IEL, and 1.14 and .007 for VCIEL. Comparable improvements with VCIEL over individual Vh:Cd and IEL were observed for other studies, including a nontherapeutic gluten challenge study.
In NCT03409796 trial data, the computation of VCIEL values showed an improved statistical significance relative to the component values of Vh:Cd and IEL by the within-group paired 2-tailed t test P values from baseline to day 15, particularly at a 10-g gluten challenge dose: Vh:Cd, IEL, VCIEL = .0050, .0031, and .0014, respectively.
Little correlation emerged between baseline values and changes with intervention for Vh:Cd and IEL on an individual patient basis.
The greater accuracy and statistical precision of the VCIEL scale are presumably due to averaging over some of the measurement uncertainty in individual patient and timepoint Vh:Cd and IEL values and creating a composite of different histologic properties, the authors noted.
This study was funded by ImmunogenX, Inc. First author Jack A. Syage is a cofounder and shareholder in ImmunogenX Inc. Dr. Silvester has served on an advisory board for Takeda Pharmaceuticals and has received research funding from Biomedal S.L., Cour Pharmaceuticals, and Glutenostics LLC. Several coauthors disclosed various financial ties to multiple private-sector pharmaceutical and biomedical companies, including ImmunogenX.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Revamped Antibiotic May Treat Deadly Eye Infection
The relatively new antibiotic cefiderocol given in the form of eye drops may be a way to combat a type of ocular infection that broke out in the United States last year, according to research presented at the 2024 annual meeting of the Association for Research in Vision and Ophthalmology (ARVO).
The infections, linked to contaminated bottles of artificial tears, were detected in 81 patients in 18 states. The outbreak led to loss of vision in 14 patients, surgical removal of the eyeball in four patients, and four deaths, according to health officials.
An extensively drug-resistant strain of Pseudomonas aeruginosa that had not previously been reported in the country caused the infections. Scientists cautioned last year that the bacteria potentially could spread from person to person.
At ARVO on May 6, Eric G. Romanowski, MS, research director of the Charles T. Campbell Ophthalmic Microbiology Laboratory at the University of Pittsburgh Medical Center, Pittsburgh, Pennsylvania, described studies that his lab conducted evaluating topical cefiderocol as a potential treatment option for these infections (Abstract 2095).
Investigators had found that the bacterial strain was susceptible to this medication, which was approved by the US Food and Drug Administration in 2019 as a treatment for complicated urinary tract infections. But the antibiotic had not been tested as an eye drop.
“We showed that the ‘Trojan-horse’ antibiotic, cefiderocol … was non-toxic and effective against the highly resistant outbreak strain in an experimental model of infection,” Dr. Romanowski and co–lead investigator Robert M. Q. Shanks, PhD, said in a statement about their research. “These results demonstrate that topical cefiderocol could be a new weapon in the ophthalmologist’s arsenal for the treatment of corneal infections caused by highly antibiotic-resistant Pseudomonas aeruginosa.”
Experimental Models
Dr. Romanowski’s group, with colleagues at the Geisel School of Medicine at Dartmouth University, Hanover, New Hampshire, used minimum inhibitory concentration testing to evaluate the effectiveness of cefiderocol against 135 isolates from eye infections. They also tested ocular toxicity and antibiotic efficacy of cefiderocol eye drops in a rabbit model of keratitis caused by the bacterial strain.
Cefiderocol was “well tolerated on rabbit corneas,” they reported. It also was effective in vitro against the isolates and in vivo in the rabbit model of keratitis.
They first published their findings as a preprint in September 2023 and then in Ophthalmology Science in December.
A ‘Duty to the Profession’
Their paper noted that “there is no current consensus as to the most effective antimicrobial strategy to deal with” extensively drug-resistant keratitis.
During the outbreak, clinicians tried various treatment regimens, with mixed results. In one case, a combination of intravenous cefiderocol and other topical and oral medications appeared to be successful.
Dr. Romanowski’s team decided to test cefiderocol drops with their own resources “as a duty to the profession,” he said. “Not many labs do these types of studies.”
“We would like to see further development of this antibiotic for potential use,” Dr. Romanowski added. “It would be up to any individual clinician to determine whether to use this antibiotic in an emergency situation.”
A version of this article appeared on Medscape.com.
The relatively new antibiotic cefiderocol given in the form of eye drops may be a way to combat a type of ocular infection that broke out in the United States last year, according to research presented at the 2024 annual meeting of the Association for Research in Vision and Ophthalmology (ARVO).
The infections, linked to contaminated bottles of artificial tears, were detected in 81 patients in 18 states. The outbreak led to loss of vision in 14 patients, surgical removal of the eyeball in four patients, and four deaths, according to health officials.
An extensively drug-resistant strain of Pseudomonas aeruginosa that had not previously been reported in the country caused the infections. Scientists cautioned last year that the bacteria potentially could spread from person to person.
At ARVO on May 6, Eric G. Romanowski, MS, research director of the Charles T. Campbell Ophthalmic Microbiology Laboratory at the University of Pittsburgh Medical Center, Pittsburgh, Pennsylvania, described studies that his lab conducted evaluating topical cefiderocol as a potential treatment option for these infections (Abstract 2095).
Investigators had found that the bacterial strain was susceptible to this medication, which was approved by the US Food and Drug Administration in 2019 as a treatment for complicated urinary tract infections. But the antibiotic had not been tested as an eye drop.
“We showed that the ‘Trojan-horse’ antibiotic, cefiderocol … was non-toxic and effective against the highly resistant outbreak strain in an experimental model of infection,” Dr. Romanowski and co–lead investigator Robert M. Q. Shanks, PhD, said in a statement about their research. “These results demonstrate that topical cefiderocol could be a new weapon in the ophthalmologist’s arsenal for the treatment of corneal infections caused by highly antibiotic-resistant Pseudomonas aeruginosa.”
Experimental Models
Dr. Romanowski’s group, with colleagues at the Geisel School of Medicine at Dartmouth University, Hanover, New Hampshire, used minimum inhibitory concentration testing to evaluate the effectiveness of cefiderocol against 135 isolates from eye infections. They also tested ocular toxicity and antibiotic efficacy of cefiderocol eye drops in a rabbit model of keratitis caused by the bacterial strain.
Cefiderocol was “well tolerated on rabbit corneas,” they reported. It also was effective in vitro against the isolates and in vivo in the rabbit model of keratitis.
They first published their findings as a preprint in September 2023 and then in Ophthalmology Science in December.
A ‘Duty to the Profession’
Their paper noted that “there is no current consensus as to the most effective antimicrobial strategy to deal with” extensively drug-resistant keratitis.
During the outbreak, clinicians tried various treatment regimens, with mixed results. In one case, a combination of intravenous cefiderocol and other topical and oral medications appeared to be successful.
Dr. Romanowski’s team decided to test cefiderocol drops with their own resources “as a duty to the profession,” he said. “Not many labs do these types of studies.”
“We would like to see further development of this antibiotic for potential use,” Dr. Romanowski added. “It would be up to any individual clinician to determine whether to use this antibiotic in an emergency situation.”
A version of this article appeared on Medscape.com.
The relatively new antibiotic cefiderocol given in the form of eye drops may be a way to combat a type of ocular infection that broke out in the United States last year, according to research presented at the 2024 annual meeting of the Association for Research in Vision and Ophthalmology (ARVO).
The infections, linked to contaminated bottles of artificial tears, were detected in 81 patients in 18 states. The outbreak led to loss of vision in 14 patients, surgical removal of the eyeball in four patients, and four deaths, according to health officials.
An extensively drug-resistant strain of Pseudomonas aeruginosa that had not previously been reported in the country caused the infections. Scientists cautioned last year that the bacteria potentially could spread from person to person.
At ARVO on May 6, Eric G. Romanowski, MS, research director of the Charles T. Campbell Ophthalmic Microbiology Laboratory at the University of Pittsburgh Medical Center, Pittsburgh, Pennsylvania, described studies that his lab conducted evaluating topical cefiderocol as a potential treatment option for these infections (Abstract 2095).
Investigators had found that the bacterial strain was susceptible to this medication, which was approved by the US Food and Drug Administration in 2019 as a treatment for complicated urinary tract infections. But the antibiotic had not been tested as an eye drop.
“We showed that the ‘Trojan-horse’ antibiotic, cefiderocol … was non-toxic and effective against the highly resistant outbreak strain in an experimental model of infection,” Dr. Romanowski and co–lead investigator Robert M. Q. Shanks, PhD, said in a statement about their research. “These results demonstrate that topical cefiderocol could be a new weapon in the ophthalmologist’s arsenal for the treatment of corneal infections caused by highly antibiotic-resistant Pseudomonas aeruginosa.”
Experimental Models
Dr. Romanowski’s group, with colleagues at the Geisel School of Medicine at Dartmouth University, Hanover, New Hampshire, used minimum inhibitory concentration testing to evaluate the effectiveness of cefiderocol against 135 isolates from eye infections. They also tested ocular toxicity and antibiotic efficacy of cefiderocol eye drops in a rabbit model of keratitis caused by the bacterial strain.
Cefiderocol was “well tolerated on rabbit corneas,” they reported. It also was effective in vitro against the isolates and in vivo in the rabbit model of keratitis.
They first published their findings as a preprint in September 2023 and then in Ophthalmology Science in December.
A ‘Duty to the Profession’
Their paper noted that “there is no current consensus as to the most effective antimicrobial strategy to deal with” extensively drug-resistant keratitis.
During the outbreak, clinicians tried various treatment regimens, with mixed results. In one case, a combination of intravenous cefiderocol and other topical and oral medications appeared to be successful.
Dr. Romanowski’s team decided to test cefiderocol drops with their own resources “as a duty to the profession,” he said. “Not many labs do these types of studies.”
“We would like to see further development of this antibiotic for potential use,” Dr. Romanowski added. “It would be up to any individual clinician to determine whether to use this antibiotic in an emergency situation.”
A version of this article appeared on Medscape.com.
Intermittent Fasting + HIIT: Fitness Fad or Fix?
Let’s be honest: Although as physicians we have the power of the prescription pad, so much of health, in the end, comes down to lifestyle. Of course, taking a pill is often way easier than changing your longstanding habits. And what’s worse, doesn’t it always seem like the lifestyle stuff that is good for your health is unpleasant?
Two recent lifestyle interventions that I have tried and find really not enjoyable are time-restricted eating (also known as intermittent fasting) and high-intensity interval training, or HIIT. The former leaves me hangry for half the day; the latter is, well, it’s just really hard compared with my usual jog.
But given the rule of unpleasant lifestyle changes, I knew as soon as I saw this recent study what the result would be. What if we combined time-restricted eating with high-intensity interval training?
I’m referring to this study, appearing in PLOS ONE from Ranya Ameur and colleagues, which is a small trial that enrolled otherwise healthy women with a BMI > 30 and randomized them to one of three conditions.
First was time-restricted eating. Women in this group could eat whatever they wanted, but only from 8 a.m. to 4 p.m. daily.
Second: high-intensity functional training. This is a variant of high-intensity interval training which focuses a bit more on resistance exercise than on pure cardiovascular stuff but has the same vibe of doing brief bursts of intensive activity followed by a cool-down period.
Third: a combination of the two. Sounds rough to me.
The study was otherwise straightforward. At baseline, researchers collected data on body composition and dietary intake, and measured blood pressure, glucose, insulin, and lipid biomarkers. A 12-week intervention period followed, after which all of this stuff was measured again.
Now, you may have noticed that there is no control group in this study. We’ll come back to that — a few times.
Let me walk you through some of the outcomes here.
First off, body composition metrics. All three groups lost weight — on average, around 10% of body weight which, for a 12-week intervention, is fairly impressive. BMI and waist circumference went down as well, and, interestingly, much of the weight loss here was in fat mass, not fat-free mass.
Most interventions that lead to weight loss — and I’m including some of the newer drugs here — lead to both fat and muscle loss. That might not be as bad as it sounds; the truth is that muscle mass increases as fat increases because of the simple fact that if you’re carrying more weight when you walk around, your leg muscles get bigger. But to preserve muscle mass in the face of fat loss is sort of a Goldilocks finding, and, based on these results, there’s a suggestion that the high-intensity functional training helps to do just that.
The dietary intake findings were really surprising to me. Across the board, caloric intake decreased. It’s no surprise that time-restricted eating reduces calorie intake. That has been shown many times before and is probably the main reason it induces weight loss — less time to eat means you eat less.
But why would high-intensity functional training lead to lower caloric intake? Most people, myself included, get hungry after they exercise. In fact, one of the reasons it’s hard to lose weight with exercise alone is that we end up eating more calories to make up for what we lost during the exercise. This calorie reduction could be a unique effect of this type of exercise, but honestly this could also be something called the Hawthorne effect. Women in the study kept a food diary to track their intake, and the act of doing that might actually make you eat less. It makes it a little more annoying to snack a bit if you know you have to write it down. This is a situation where I would kill for a control group.
The lipid findings are also pretty striking, with around a 40% reduction in LDL across the board, and evidence of synergistic effects of combined TRE and high-intensity training on total cholesterol and triglycerides. This is like a statin level of effect — pretty impressive. Again, my heart pines for a control group, though.
Same story with glucose and insulin measures: an impressive reduction in fasting glucose and good evidence that the combination of time-restricted eating and high-intensity functional training reduces insulin levels and HOMA-IR as well.
Really the only thing that wasn’t very impressive was the change in blood pressure, with only modest decreases across the board.
Okay, so let’s take a breath after this high-intensity cerebral workout and put this all together. This was a small study, lacking a control group, but with large effect sizes in very relevant clinical areas. It confirms what we know about time-restricted eating — that it makes you eat less calories — and introduces the potential that vigorous exercise can not only magnify the benefits of time-restricted eating but maybe even mitigate some of the risks, like the risk for muscle loss. And of course, it comports with my central hypothesis, which is that the more unpleasant a lifestyle intervention is, the better it is for you. No pain, no gain, right?
Of course, I am being overly dogmatic. There are plenty of caveats. Wrestling bears is quite unpleasant and almost certainly bad for you. And there are even some pleasant things that are pretty good for you — like coffee and sex. And there are even people who find time-restricted eating and high-intensity training pleasurable. They are called masochists.
I’m joking. The truth is that Or, at least, much less painful. The trick is getting over the hump of change. If only there were a pill for that.
Dr. Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Connecticut. He has disclosed no relevant financial relationships. This transcript has been edited for clarity.
A version of this article appeared on Medscape.com.
Let’s be honest: Although as physicians we have the power of the prescription pad, so much of health, in the end, comes down to lifestyle. Of course, taking a pill is often way easier than changing your longstanding habits. And what’s worse, doesn’t it always seem like the lifestyle stuff that is good for your health is unpleasant?
Two recent lifestyle interventions that I have tried and find really not enjoyable are time-restricted eating (also known as intermittent fasting) and high-intensity interval training, or HIIT. The former leaves me hangry for half the day; the latter is, well, it’s just really hard compared with my usual jog.
But given the rule of unpleasant lifestyle changes, I knew as soon as I saw this recent study what the result would be. What if we combined time-restricted eating with high-intensity interval training?
I’m referring to this study, appearing in PLOS ONE from Ranya Ameur and colleagues, which is a small trial that enrolled otherwise healthy women with a BMI > 30 and randomized them to one of three conditions.
First was time-restricted eating. Women in this group could eat whatever they wanted, but only from 8 a.m. to 4 p.m. daily.
Second: high-intensity functional training. This is a variant of high-intensity interval training which focuses a bit more on resistance exercise than on pure cardiovascular stuff but has the same vibe of doing brief bursts of intensive activity followed by a cool-down period.
Third: a combination of the two. Sounds rough to me.
The study was otherwise straightforward. At baseline, researchers collected data on body composition and dietary intake, and measured blood pressure, glucose, insulin, and lipid biomarkers. A 12-week intervention period followed, after which all of this stuff was measured again.
Now, you may have noticed that there is no control group in this study. We’ll come back to that — a few times.
Let me walk you through some of the outcomes here.
First off, body composition metrics. All three groups lost weight — on average, around 10% of body weight which, for a 12-week intervention, is fairly impressive. BMI and waist circumference went down as well, and, interestingly, much of the weight loss here was in fat mass, not fat-free mass.
Most interventions that lead to weight loss — and I’m including some of the newer drugs here — lead to both fat and muscle loss. That might not be as bad as it sounds; the truth is that muscle mass increases as fat increases because of the simple fact that if you’re carrying more weight when you walk around, your leg muscles get bigger. But to preserve muscle mass in the face of fat loss is sort of a Goldilocks finding, and, based on these results, there’s a suggestion that the high-intensity functional training helps to do just that.
The dietary intake findings were really surprising to me. Across the board, caloric intake decreased. It’s no surprise that time-restricted eating reduces calorie intake. That has been shown many times before and is probably the main reason it induces weight loss — less time to eat means you eat less.
But why would high-intensity functional training lead to lower caloric intake? Most people, myself included, get hungry after they exercise. In fact, one of the reasons it’s hard to lose weight with exercise alone is that we end up eating more calories to make up for what we lost during the exercise. This calorie reduction could be a unique effect of this type of exercise, but honestly this could also be something called the Hawthorne effect. Women in the study kept a food diary to track their intake, and the act of doing that might actually make you eat less. It makes it a little more annoying to snack a bit if you know you have to write it down. This is a situation where I would kill for a control group.
The lipid findings are also pretty striking, with around a 40% reduction in LDL across the board, and evidence of synergistic effects of combined TRE and high-intensity training on total cholesterol and triglycerides. This is like a statin level of effect — pretty impressive. Again, my heart pines for a control group, though.
Same story with glucose and insulin measures: an impressive reduction in fasting glucose and good evidence that the combination of time-restricted eating and high-intensity functional training reduces insulin levels and HOMA-IR as well.
Really the only thing that wasn’t very impressive was the change in blood pressure, with only modest decreases across the board.
Okay, so let’s take a breath after this high-intensity cerebral workout and put this all together. This was a small study, lacking a control group, but with large effect sizes in very relevant clinical areas. It confirms what we know about time-restricted eating — that it makes you eat less calories — and introduces the potential that vigorous exercise can not only magnify the benefits of time-restricted eating but maybe even mitigate some of the risks, like the risk for muscle loss. And of course, it comports with my central hypothesis, which is that the more unpleasant a lifestyle intervention is, the better it is for you. No pain, no gain, right?
Of course, I am being overly dogmatic. There are plenty of caveats. Wrestling bears is quite unpleasant and almost certainly bad for you. And there are even some pleasant things that are pretty good for you — like coffee and sex. And there are even people who find time-restricted eating and high-intensity training pleasurable. They are called masochists.
I’m joking. The truth is that Or, at least, much less painful. The trick is getting over the hump of change. If only there were a pill for that.
Dr. Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Connecticut. He has disclosed no relevant financial relationships. This transcript has been edited for clarity.
A version of this article appeared on Medscape.com.
Let’s be honest: Although as physicians we have the power of the prescription pad, so much of health, in the end, comes down to lifestyle. Of course, taking a pill is often way easier than changing your longstanding habits. And what’s worse, doesn’t it always seem like the lifestyle stuff that is good for your health is unpleasant?
Two recent lifestyle interventions that I have tried and find really not enjoyable are time-restricted eating (also known as intermittent fasting) and high-intensity interval training, or HIIT. The former leaves me hangry for half the day; the latter is, well, it’s just really hard compared with my usual jog.
But given the rule of unpleasant lifestyle changes, I knew as soon as I saw this recent study what the result would be. What if we combined time-restricted eating with high-intensity interval training?
I’m referring to this study, appearing in PLOS ONE from Ranya Ameur and colleagues, which is a small trial that enrolled otherwise healthy women with a BMI > 30 and randomized them to one of three conditions.
First was time-restricted eating. Women in this group could eat whatever they wanted, but only from 8 a.m. to 4 p.m. daily.
Second: high-intensity functional training. This is a variant of high-intensity interval training which focuses a bit more on resistance exercise than on pure cardiovascular stuff but has the same vibe of doing brief bursts of intensive activity followed by a cool-down period.
Third: a combination of the two. Sounds rough to me.
The study was otherwise straightforward. At baseline, researchers collected data on body composition and dietary intake, and measured blood pressure, glucose, insulin, and lipid biomarkers. A 12-week intervention period followed, after which all of this stuff was measured again.
Now, you may have noticed that there is no control group in this study. We’ll come back to that — a few times.
Let me walk you through some of the outcomes here.
First off, body composition metrics. All three groups lost weight — on average, around 10% of body weight which, for a 12-week intervention, is fairly impressive. BMI and waist circumference went down as well, and, interestingly, much of the weight loss here was in fat mass, not fat-free mass.
Most interventions that lead to weight loss — and I’m including some of the newer drugs here — lead to both fat and muscle loss. That might not be as bad as it sounds; the truth is that muscle mass increases as fat increases because of the simple fact that if you’re carrying more weight when you walk around, your leg muscles get bigger. But to preserve muscle mass in the face of fat loss is sort of a Goldilocks finding, and, based on these results, there’s a suggestion that the high-intensity functional training helps to do just that.
The dietary intake findings were really surprising to me. Across the board, caloric intake decreased. It’s no surprise that time-restricted eating reduces calorie intake. That has been shown many times before and is probably the main reason it induces weight loss — less time to eat means you eat less.
But why would high-intensity functional training lead to lower caloric intake? Most people, myself included, get hungry after they exercise. In fact, one of the reasons it’s hard to lose weight with exercise alone is that we end up eating more calories to make up for what we lost during the exercise. This calorie reduction could be a unique effect of this type of exercise, but honestly this could also be something called the Hawthorne effect. Women in the study kept a food diary to track their intake, and the act of doing that might actually make you eat less. It makes it a little more annoying to snack a bit if you know you have to write it down. This is a situation where I would kill for a control group.
The lipid findings are also pretty striking, with around a 40% reduction in LDL across the board, and evidence of synergistic effects of combined TRE and high-intensity training on total cholesterol and triglycerides. This is like a statin level of effect — pretty impressive. Again, my heart pines for a control group, though.
Same story with glucose and insulin measures: an impressive reduction in fasting glucose and good evidence that the combination of time-restricted eating and high-intensity functional training reduces insulin levels and HOMA-IR as well.
Really the only thing that wasn’t very impressive was the change in blood pressure, with only modest decreases across the board.
Okay, so let’s take a breath after this high-intensity cerebral workout and put this all together. This was a small study, lacking a control group, but with large effect sizes in very relevant clinical areas. It confirms what we know about time-restricted eating — that it makes you eat less calories — and introduces the potential that vigorous exercise can not only magnify the benefits of time-restricted eating but maybe even mitigate some of the risks, like the risk for muscle loss. And of course, it comports with my central hypothesis, which is that the more unpleasant a lifestyle intervention is, the better it is for you. No pain, no gain, right?
Of course, I am being overly dogmatic. There are plenty of caveats. Wrestling bears is quite unpleasant and almost certainly bad for you. And there are even some pleasant things that are pretty good for you — like coffee and sex. And there are even people who find time-restricted eating and high-intensity training pleasurable. They are called masochists.
I’m joking. The truth is that Or, at least, much less painful. The trick is getting over the hump of change. If only there were a pill for that.
Dr. Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Connecticut. He has disclosed no relevant financial relationships. This transcript has been edited for clarity.
A version of this article appeared on Medscape.com.
Diabetes/Weight Loss Med Linked to Repeat Spinal Surgery
CHICAGO — The diabetes/weight loss drug semaglutide is associated with a significantly greater risk for repeat operations in patients with diabetes who require lumbar surgery, a new study suggests.
The risk for additional surgeries was even higher among patients taking the popular weight loss and diabetes drug for longer periods of time.
Investigators say the study provides the first evidence on the impact of semaglutide on spine surgery.
“The expectation was [that] we would see patients doing better after surgery, less wound complications, and other things, and in our diabetic patients we did not see that and saw increased odds of needing additional surgeries,” investigator Syed I. Khalid, MD, neurosurgery resident at University of Illinois Chicago, told this news organization.
The findings were presented on May 3 at the American Association of Neurological Surgeons (AANS) 2024 Annual Meeting.
Additional Surgery at Year 1
The new study used the all-payer Mariner database to identify patients aged 18-74 years with type 2 diabetes who underwent elective one- to three-level transforaminal lumbar interbody fusions (TLIFs) between January 2018 and October 2022.
Patients were matched in a 3:1 ratio for age, sex, hypertension, obesity, smoking history, chronic kidney disease, osteoporosis, insulin use, and spinal fusion level, resulting in 447 patients with semaglutide use and 1334 with no semaglutide use. More than half (56%) were female, 62% used insulin, and 81% underwent single-level TLIF.
Total medical complications were higher in the semaglutide group, at 13.4%, compared with 7.7% in the no-semaglutide group (odds ratio [OR], 1.85). This was driven by higher rates of urinary tract infection (6.7% vs 2.5%) and acute kidney injury (6.3% vs 3.9%), two complications observed with semaglutide in other studies, Dr. Khalid said.
Total surgical complications, however, were lower in patients taking semaglutide, at 3.8% vs 5.2% in those who did not (OR, 0.73).
Patients taking semaglutide vs those who were not using semaglutide had fewer wound healing complications (5 vs 31), hematoma (1 vs 9), surgical-site infections (12 vs 44), and cerebrospinal fluid leaks (2 vs 3).
Still, people taking semaglutide were nearly 12 times more likely to have an additional lumbar surgery at 1 year than did those who did not use the drug (27.3% vs 3.1%; OR, 11.79; 95% CI, 8.17-17.33).
Kaplan-Meier plots revealed a striking divergence of these populations when semaglutide exposure for more than or less than 9 months was examined (log-rank P < .0001).
Currently under review for publication, this study provides the first evidence on the impact of semaglutide on spine surgery, Dr. Khalid said. A second follow-up paper, also under review, looked only at patients with patients morbidly obesity without diabetes who had taken semaglutide for weight loss.
“In nondiabetic, morbidly obese patients undergoing spine surgery, we see a similar trend,” Dr. Khalid said.
Sarcopenia the Cause?
The additional surgeries were primarily extensions of constructs, with additional surgery and fusion at more levels, Dr. Khalid noted.
“The idea is that it could be the fact there is sarcopenia or muscle loss that’s taking place in conjunction with fat loss that’s causing that to happen,” Dr. Khalid said.
The mechanism remains speculative, but evidence from other areas examining frailty states has shown that those patients have weaker bones, sarcopenia, and worse outcomes with spine surgery, he noted.
The investigators plan to use artificial intelligence to evaluate changes in body composition after semaglutide use in patients who underwent imaging prior to spine surgery or even before back pain occurred. Because these medications are uptitrated over time, follow-up studies will also look at whether this change takes place with a certain dose, Dr. Khalid added.
On the basis of the current analysis of generic semaglutide alone, it’s not possible to say whether the use of other glucagon-like peptide 1 (GLP-1) receptor agonists will result in similar findings, but “the odds of a class effect are high,” Dr. Khalid said.
Commenting on the findings, Walavan Sivakumar, MD, director of neurosurgery at Pacific Neuroscience Institute, Los Angeles, noted that the timing of surgery is already an issue for patients taking semaglutide and other GLP-1 receptor agonists following recent guidance from the American Society of Anesthesiologists that suggests stopping GLP-1 receptor agonists prior to elective surgery to reduce the risk for complications associated with anesthesia.
“It’s an incredibly topical point and seems to be something showing up on a daily basis for clinicians all throughout neurosurgery,” Dr. Sivakumar said. “It’s thought-provoking and a great first start.”
Dr. Sivakumar also observed that frailty is a hot topic in all of neurosurgery. “That’s a major, major point that’s showing an impact on all surgical outcomes and it’s being heavily studied in the neurosurgical subsets right now. So that’s definitely a possible correlating factor.”
Dr. Khalid reported no financial relationships. Dr. Sivakumar reported serving as a consultant for Stryker.
A version of this article appeared on Medscape.com.
CHICAGO — The diabetes/weight loss drug semaglutide is associated with a significantly greater risk for repeat operations in patients with diabetes who require lumbar surgery, a new study suggests.
The risk for additional surgeries was even higher among patients taking the popular weight loss and diabetes drug for longer periods of time.
Investigators say the study provides the first evidence on the impact of semaglutide on spine surgery.
“The expectation was [that] we would see patients doing better after surgery, less wound complications, and other things, and in our diabetic patients we did not see that and saw increased odds of needing additional surgeries,” investigator Syed I. Khalid, MD, neurosurgery resident at University of Illinois Chicago, told this news organization.
The findings were presented on May 3 at the American Association of Neurological Surgeons (AANS) 2024 Annual Meeting.
Additional Surgery at Year 1
The new study used the all-payer Mariner database to identify patients aged 18-74 years with type 2 diabetes who underwent elective one- to three-level transforaminal lumbar interbody fusions (TLIFs) between January 2018 and October 2022.
Patients were matched in a 3:1 ratio for age, sex, hypertension, obesity, smoking history, chronic kidney disease, osteoporosis, insulin use, and spinal fusion level, resulting in 447 patients with semaglutide use and 1334 with no semaglutide use. More than half (56%) were female, 62% used insulin, and 81% underwent single-level TLIF.
Total medical complications were higher in the semaglutide group, at 13.4%, compared with 7.7% in the no-semaglutide group (odds ratio [OR], 1.85). This was driven by higher rates of urinary tract infection (6.7% vs 2.5%) and acute kidney injury (6.3% vs 3.9%), two complications observed with semaglutide in other studies, Dr. Khalid said.
Total surgical complications, however, were lower in patients taking semaglutide, at 3.8% vs 5.2% in those who did not (OR, 0.73).
Patients taking semaglutide vs those who were not using semaglutide had fewer wound healing complications (5 vs 31), hematoma (1 vs 9), surgical-site infections (12 vs 44), and cerebrospinal fluid leaks (2 vs 3).
Still, people taking semaglutide were nearly 12 times more likely to have an additional lumbar surgery at 1 year than did those who did not use the drug (27.3% vs 3.1%; OR, 11.79; 95% CI, 8.17-17.33).
Kaplan-Meier plots revealed a striking divergence of these populations when semaglutide exposure for more than or less than 9 months was examined (log-rank P < .0001).
Currently under review for publication, this study provides the first evidence on the impact of semaglutide on spine surgery, Dr. Khalid said. A second follow-up paper, also under review, looked only at patients with patients morbidly obesity without diabetes who had taken semaglutide for weight loss.
“In nondiabetic, morbidly obese patients undergoing spine surgery, we see a similar trend,” Dr. Khalid said.
Sarcopenia the Cause?
The additional surgeries were primarily extensions of constructs, with additional surgery and fusion at more levels, Dr. Khalid noted.
“The idea is that it could be the fact there is sarcopenia or muscle loss that’s taking place in conjunction with fat loss that’s causing that to happen,” Dr. Khalid said.
The mechanism remains speculative, but evidence from other areas examining frailty states has shown that those patients have weaker bones, sarcopenia, and worse outcomes with spine surgery, he noted.
The investigators plan to use artificial intelligence to evaluate changes in body composition after semaglutide use in patients who underwent imaging prior to spine surgery or even before back pain occurred. Because these medications are uptitrated over time, follow-up studies will also look at whether this change takes place with a certain dose, Dr. Khalid added.
On the basis of the current analysis of generic semaglutide alone, it’s not possible to say whether the use of other glucagon-like peptide 1 (GLP-1) receptor agonists will result in similar findings, but “the odds of a class effect are high,” Dr. Khalid said.
Commenting on the findings, Walavan Sivakumar, MD, director of neurosurgery at Pacific Neuroscience Institute, Los Angeles, noted that the timing of surgery is already an issue for patients taking semaglutide and other GLP-1 receptor agonists following recent guidance from the American Society of Anesthesiologists that suggests stopping GLP-1 receptor agonists prior to elective surgery to reduce the risk for complications associated with anesthesia.
“It’s an incredibly topical point and seems to be something showing up on a daily basis for clinicians all throughout neurosurgery,” Dr. Sivakumar said. “It’s thought-provoking and a great first start.”
Dr. Sivakumar also observed that frailty is a hot topic in all of neurosurgery. “That’s a major, major point that’s showing an impact on all surgical outcomes and it’s being heavily studied in the neurosurgical subsets right now. So that’s definitely a possible correlating factor.”
Dr. Khalid reported no financial relationships. Dr. Sivakumar reported serving as a consultant for Stryker.
A version of this article appeared on Medscape.com.
CHICAGO — The diabetes/weight loss drug semaglutide is associated with a significantly greater risk for repeat operations in patients with diabetes who require lumbar surgery, a new study suggests.
The risk for additional surgeries was even higher among patients taking the popular weight loss and diabetes drug for longer periods of time.
Investigators say the study provides the first evidence on the impact of semaglutide on spine surgery.
“The expectation was [that] we would see patients doing better after surgery, less wound complications, and other things, and in our diabetic patients we did not see that and saw increased odds of needing additional surgeries,” investigator Syed I. Khalid, MD, neurosurgery resident at University of Illinois Chicago, told this news organization.
The findings were presented on May 3 at the American Association of Neurological Surgeons (AANS) 2024 Annual Meeting.
Additional Surgery at Year 1
The new study used the all-payer Mariner database to identify patients aged 18-74 years with type 2 diabetes who underwent elective one- to three-level transforaminal lumbar interbody fusions (TLIFs) between January 2018 and October 2022.
Patients were matched in a 3:1 ratio for age, sex, hypertension, obesity, smoking history, chronic kidney disease, osteoporosis, insulin use, and spinal fusion level, resulting in 447 patients with semaglutide use and 1334 with no semaglutide use. More than half (56%) were female, 62% used insulin, and 81% underwent single-level TLIF.
Total medical complications were higher in the semaglutide group, at 13.4%, compared with 7.7% in the no-semaglutide group (odds ratio [OR], 1.85). This was driven by higher rates of urinary tract infection (6.7% vs 2.5%) and acute kidney injury (6.3% vs 3.9%), two complications observed with semaglutide in other studies, Dr. Khalid said.
Total surgical complications, however, were lower in patients taking semaglutide, at 3.8% vs 5.2% in those who did not (OR, 0.73).
Patients taking semaglutide vs those who were not using semaglutide had fewer wound healing complications (5 vs 31), hematoma (1 vs 9), surgical-site infections (12 vs 44), and cerebrospinal fluid leaks (2 vs 3).
Still, people taking semaglutide were nearly 12 times more likely to have an additional lumbar surgery at 1 year than did those who did not use the drug (27.3% vs 3.1%; OR, 11.79; 95% CI, 8.17-17.33).
Kaplan-Meier plots revealed a striking divergence of these populations when semaglutide exposure for more than or less than 9 months was examined (log-rank P < .0001).
Currently under review for publication, this study provides the first evidence on the impact of semaglutide on spine surgery, Dr. Khalid said. A second follow-up paper, also under review, looked only at patients with patients morbidly obesity without diabetes who had taken semaglutide for weight loss.
“In nondiabetic, morbidly obese patients undergoing spine surgery, we see a similar trend,” Dr. Khalid said.
Sarcopenia the Cause?
The additional surgeries were primarily extensions of constructs, with additional surgery and fusion at more levels, Dr. Khalid noted.
“The idea is that it could be the fact there is sarcopenia or muscle loss that’s taking place in conjunction with fat loss that’s causing that to happen,” Dr. Khalid said.
The mechanism remains speculative, but evidence from other areas examining frailty states has shown that those patients have weaker bones, sarcopenia, and worse outcomes with spine surgery, he noted.
The investigators plan to use artificial intelligence to evaluate changes in body composition after semaglutide use in patients who underwent imaging prior to spine surgery or even before back pain occurred. Because these medications are uptitrated over time, follow-up studies will also look at whether this change takes place with a certain dose, Dr. Khalid added.
On the basis of the current analysis of generic semaglutide alone, it’s not possible to say whether the use of other glucagon-like peptide 1 (GLP-1) receptor agonists will result in similar findings, but “the odds of a class effect are high,” Dr. Khalid said.
Commenting on the findings, Walavan Sivakumar, MD, director of neurosurgery at Pacific Neuroscience Institute, Los Angeles, noted that the timing of surgery is already an issue for patients taking semaglutide and other GLP-1 receptor agonists following recent guidance from the American Society of Anesthesiologists that suggests stopping GLP-1 receptor agonists prior to elective surgery to reduce the risk for complications associated with anesthesia.
“It’s an incredibly topical point and seems to be something showing up on a daily basis for clinicians all throughout neurosurgery,” Dr. Sivakumar said. “It’s thought-provoking and a great first start.”
Dr. Sivakumar also observed that frailty is a hot topic in all of neurosurgery. “That’s a major, major point that’s showing an impact on all surgical outcomes and it’s being heavily studied in the neurosurgical subsets right now. So that’s definitely a possible correlating factor.”
Dr. Khalid reported no financial relationships. Dr. Sivakumar reported serving as a consultant for Stryker.
A version of this article appeared on Medscape.com.
Diacerein, Resveratrol, Botulinum Toxin Disappoint in Knee Osteoarthritis
VIENNA — Data do not back the use of diacerein or resveratrol for managing the pain of knee osteoarthritis (OA), according to the results of two well-performed, multicenter, double-blind, randomized controlled clinical trials.
During the News in Therapies session at the OARSI 2024 World Congress, the null findings of the DICKENS study and ARTHROL trial were presented alongside a reappraisal of the possible role of botulinum toxin.
DICKENS Study of Diacerein
“The role of diacerein in the treatment of OA is controversial,” acknowledged Dawn Aitken, PhD, associate professor at the University of Tasmania in Hobart, Tasmania, Australia. “There are only a few acceptable quality trials to date, and the results are inconsistent,” Dr. Aitken added.
Indeed, a Cochrane review performed in 2014 had concluded that there was “low-quality evidence that diacerein had a small beneficial effect on pain,” she said. The reported overall effect size on a 100-mm visual analog scale, based on a meta-analysis of 10 trials, has been just −8.65 mm, equating to just a 9% pain reduction.
At the time the DICKENS study was conceived, diacerein was recommended by a number of international guidelines for the management of hip and knee OA, although further, higher-quality studies were needed.
Diacerein blocks interleukin-1 beta, which is one of the key inflammatory markers of OA, so Dr. Aitken and collaborators postulated that perhaps it would work better if used in patients with an inflammatory phenotype.
They set about to test their hypothesis by recruiting 260 individuals with knee OA and MRI-detected effusion synovitis. The participants were then randomly allocated to treatment with either diacerein or a matching placebo for 24 weeks.
Individuals in the diacerein group were treated with an oral dose of 50 mg once daily for the first 2 weeks. If tolerated, the dose was increased to 50 mg twice daily.
No significant improvement in the primary endpoint of knee pain was seen comparing diacerein with placebo, with mean values of 53.2 mm and 56.4 mm, respectively, at 24 weeks using a 0-100 mm visual analog scale where 0 represented no pain and 100 represented the worst pain. It followed that there was no significant difference in the change from baseline to week 24 (−19.9 mm vs −18.6 mm; P = .77).
There was also no difference in the secondary endpoints, which included Western Ontario and McMaster Universities Arthritis Index pain, function, and stiffness. In fact, placebo-treated patients appeared to do better in terms of resolution of effusion synovitis as measured by a repeat MRI and quality of life, Dr. Aitken reported.
“These findings do not support the use of diacerein in treating patients with knee OA and effusion synovitis,” Dr. Aitken concluded.
ARTHROL Trial of Resveratrol
Similarly, negative results were reported for resveratrol from the ARTHROL trial, with 55% of the resveratrol- and 55% of placebo-treated individuals achieving a 20% reduction in knee pain intensity at 3 months. The actual change in knee pain from baseline to 3 months was −15.7 for resveratrol and −15.2 for placebo on a numerical rating scale that went from 0 (no pain) up to 100 (worst pain).
Resveratrol is found naturally in grapes, peanuts, pine cones, and Chinese knotweed, and there is a growing body of evidence that it may have pleiotropic effects, said investigator Christelle Nguyen, PhD, MD, a professor of physical and rehabilitation medicine at Université Paris Cité, Paris, France.
It’s available in a powder form over the counter as a treatment for multiple ailments, but more recently, became available as an oral formulation. Dr. Nguyen and colleagues wanted to know if this would make a difference to OA knee pain when added to usual care.
A double-blind, multicenter, placebo-controlled randomized trial was therefore conducted that involved 142 people with knee OA who had been experiencing knee pain for at least 1 month. The participants were equally randomly allocated to receive either oral resveratrol given as two caplets of 20 mg twice daily for the first week, then once daily for a total of 6 months, or a matched placebo.
There was also no effect of resveratrol vs placebo on a host of secondary outcomes measured at 3 and 6 months.
The interpretation is that oral resveratrol may not be effective in this indication or have a biologic effect on the pain pathway, Dr. Nguyen said.
“Our findings do not support the use of [trans-resveratrol] supplementation in this patented formulation for reducing knee pain in adults with painful knee OA,” she concluded.
Botulinum Toxin: Over But Not Out?
Dr. Nguyen separately reported data from a new systematic review and meta-analysis on the use of intra-articular (IA) botulinum toxin type A (BoNT-A) for knee OA pain.
Seven of the 14 randomized controlled trials included in the meta-analysis had looked specifically at knee OA outcomes in the short, intermediate, and long term.
Results showed a nonsignificant trend favoring BoNT-A use, with the standard mean difference in pain of 0.35 (−0.82; 0.12), −0.27 (−0.61; 0.08), and −0.43 (−1.12; 0.26) for short-, intermediate-, and long-term use, respectively.
In contrast, pain reductions were seen with BoNT-A in three trials that included people with OA of the shoulder or base of the thumb. This begs the question as to whether botulinum toxin may still have a role to play, Dr. Nguyen said in an interview.
“It seems like there may be a positive effect for the shoulder joint and base of the thumb,” she told this news organization.
“So, basically, we found differences between large and small to intermediate joints,” Dr. Nguyen added. “It questions the dilution of botulinum toxin into the joint. If it’s a big joint, maybe the dilution is too high,” she suggested.
This hypothesis will be tested in the upcoming RHIBOT II trial that will begin recruitment later this year. This is a follow-on from the RHIBOT trial that was published in The Lancet Rheumatology 2 years ago.
Meanwhile, the use of botulinum toxin is off-label, Dr. Nguyen said. “We use it in our clinics only when first-line treatment had failed for base of thumb OA.” It’s not offered as a stand-alone intervention, and the IA injections need to be given by someone with experience, she said.
Methodologically Sound Studies
Commenting on the studies, Nancy E. Lane, MD, said: “There have been small botulinum studies before but not powered enough so that you could confirm or refute hypotheses.”
Dr. Lane, endowed professor of medicine, rheumatology, and aging research and director for the Center for Musculoskeletal Health at the University of California Davis School of Medicine, Sacramento, California, added: “Similarly for resveratrol, there have been lots of studies.”
Moreover, Dr. Lane observed that the studies were “really well-designed. They were well-powered. The subjects were selected in such a way that was good rigor in the methodologic design, and there were enough people in the studies so that you could really believe the results.”
The take-home is probably that these approaches do not work, Dr. Lane said, “at least when you apply them to moderate-severe knee OA patients, they don’t seem to make a difference.”
The congress was sponsored by the Osteoarthritis Research Society International.
The DICKENS study of diacerein was an investigator-initiated trial that was funded by the National Health and Medical Research Council of Australia. TRB Chemedica International S.A. provided diacerein free of charge for the trial but was not involved in the implementation or data analysis. Dr. Aitken had no conflicts of interest to disclose.
The ARTHROL trial of oral resveratrol was funded by the French Ministry of Health and Solidarity (Ministré des Solidarités et de la Santé). Yvery Laboratory provided the resveratrol caplet and matching placebo free of charge. Dr. Nguyen has financial relationships with Actelion, Grünenthal, Ipsen, Lilly, Meda, Merz, Novartis, Preciphar, Sandoz, Takeda, Thuasne, and UCB.
Dr. Lane had no relevant conflicts of interest to declare.
A version of this article appeared on Medscape.com.
VIENNA — Data do not back the use of diacerein or resveratrol for managing the pain of knee osteoarthritis (OA), according to the results of two well-performed, multicenter, double-blind, randomized controlled clinical trials.
During the News in Therapies session at the OARSI 2024 World Congress, the null findings of the DICKENS study and ARTHROL trial were presented alongside a reappraisal of the possible role of botulinum toxin.
DICKENS Study of Diacerein
“The role of diacerein in the treatment of OA is controversial,” acknowledged Dawn Aitken, PhD, associate professor at the University of Tasmania in Hobart, Tasmania, Australia. “There are only a few acceptable quality trials to date, and the results are inconsistent,” Dr. Aitken added.
Indeed, a Cochrane review performed in 2014 had concluded that there was “low-quality evidence that diacerein had a small beneficial effect on pain,” she said. The reported overall effect size on a 100-mm visual analog scale, based on a meta-analysis of 10 trials, has been just −8.65 mm, equating to just a 9% pain reduction.
At the time the DICKENS study was conceived, diacerein was recommended by a number of international guidelines for the management of hip and knee OA, although further, higher-quality studies were needed.
Diacerein blocks interleukin-1 beta, which is one of the key inflammatory markers of OA, so Dr. Aitken and collaborators postulated that perhaps it would work better if used in patients with an inflammatory phenotype.
They set about to test their hypothesis by recruiting 260 individuals with knee OA and MRI-detected effusion synovitis. The participants were then randomly allocated to treatment with either diacerein or a matching placebo for 24 weeks.
Individuals in the diacerein group were treated with an oral dose of 50 mg once daily for the first 2 weeks. If tolerated, the dose was increased to 50 mg twice daily.
No significant improvement in the primary endpoint of knee pain was seen comparing diacerein with placebo, with mean values of 53.2 mm and 56.4 mm, respectively, at 24 weeks using a 0-100 mm visual analog scale where 0 represented no pain and 100 represented the worst pain. It followed that there was no significant difference in the change from baseline to week 24 (−19.9 mm vs −18.6 mm; P = .77).
There was also no difference in the secondary endpoints, which included Western Ontario and McMaster Universities Arthritis Index pain, function, and stiffness. In fact, placebo-treated patients appeared to do better in terms of resolution of effusion synovitis as measured by a repeat MRI and quality of life, Dr. Aitken reported.
“These findings do not support the use of diacerein in treating patients with knee OA and effusion synovitis,” Dr. Aitken concluded.
ARTHROL Trial of Resveratrol
Similarly, negative results were reported for resveratrol from the ARTHROL trial, with 55% of the resveratrol- and 55% of placebo-treated individuals achieving a 20% reduction in knee pain intensity at 3 months. The actual change in knee pain from baseline to 3 months was −15.7 for resveratrol and −15.2 for placebo on a numerical rating scale that went from 0 (no pain) up to 100 (worst pain).
Resveratrol is found naturally in grapes, peanuts, pine cones, and Chinese knotweed, and there is a growing body of evidence that it may have pleiotropic effects, said investigator Christelle Nguyen, PhD, MD, a professor of physical and rehabilitation medicine at Université Paris Cité, Paris, France.
It’s available in a powder form over the counter as a treatment for multiple ailments, but more recently, became available as an oral formulation. Dr. Nguyen and colleagues wanted to know if this would make a difference to OA knee pain when added to usual care.
A double-blind, multicenter, placebo-controlled randomized trial was therefore conducted that involved 142 people with knee OA who had been experiencing knee pain for at least 1 month. The participants were equally randomly allocated to receive either oral resveratrol given as two caplets of 20 mg twice daily for the first week, then once daily for a total of 6 months, or a matched placebo.
There was also no effect of resveratrol vs placebo on a host of secondary outcomes measured at 3 and 6 months.
The interpretation is that oral resveratrol may not be effective in this indication or have a biologic effect on the pain pathway, Dr. Nguyen said.
“Our findings do not support the use of [trans-resveratrol] supplementation in this patented formulation for reducing knee pain in adults with painful knee OA,” she concluded.
Botulinum Toxin: Over But Not Out?
Dr. Nguyen separately reported data from a new systematic review and meta-analysis on the use of intra-articular (IA) botulinum toxin type A (BoNT-A) for knee OA pain.
Seven of the 14 randomized controlled trials included in the meta-analysis had looked specifically at knee OA outcomes in the short, intermediate, and long term.
Results showed a nonsignificant trend favoring BoNT-A use, with the standard mean difference in pain of 0.35 (−0.82; 0.12), −0.27 (−0.61; 0.08), and −0.43 (−1.12; 0.26) for short-, intermediate-, and long-term use, respectively.
In contrast, pain reductions were seen with BoNT-A in three trials that included people with OA of the shoulder or base of the thumb. This begs the question as to whether botulinum toxin may still have a role to play, Dr. Nguyen said in an interview.
“It seems like there may be a positive effect for the shoulder joint and base of the thumb,” she told this news organization.
“So, basically, we found differences between large and small to intermediate joints,” Dr. Nguyen added. “It questions the dilution of botulinum toxin into the joint. If it’s a big joint, maybe the dilution is too high,” she suggested.
This hypothesis will be tested in the upcoming RHIBOT II trial that will begin recruitment later this year. This is a follow-on from the RHIBOT trial that was published in The Lancet Rheumatology 2 years ago.
Meanwhile, the use of botulinum toxin is off-label, Dr. Nguyen said. “We use it in our clinics only when first-line treatment had failed for base of thumb OA.” It’s not offered as a stand-alone intervention, and the IA injections need to be given by someone with experience, she said.
Methodologically Sound Studies
Commenting on the studies, Nancy E. Lane, MD, said: “There have been small botulinum studies before but not powered enough so that you could confirm or refute hypotheses.”
Dr. Lane, endowed professor of medicine, rheumatology, and aging research and director for the Center for Musculoskeletal Health at the University of California Davis School of Medicine, Sacramento, California, added: “Similarly for resveratrol, there have been lots of studies.”
Moreover, Dr. Lane observed that the studies were “really well-designed. They were well-powered. The subjects were selected in such a way that was good rigor in the methodologic design, and there were enough people in the studies so that you could really believe the results.”
The take-home is probably that these approaches do not work, Dr. Lane said, “at least when you apply them to moderate-severe knee OA patients, they don’t seem to make a difference.”
The congress was sponsored by the Osteoarthritis Research Society International.
The DICKENS study of diacerein was an investigator-initiated trial that was funded by the National Health and Medical Research Council of Australia. TRB Chemedica International S.A. provided diacerein free of charge for the trial but was not involved in the implementation or data analysis. Dr. Aitken had no conflicts of interest to disclose.
The ARTHROL trial of oral resveratrol was funded by the French Ministry of Health and Solidarity (Ministré des Solidarités et de la Santé). Yvery Laboratory provided the resveratrol caplet and matching placebo free of charge. Dr. Nguyen has financial relationships with Actelion, Grünenthal, Ipsen, Lilly, Meda, Merz, Novartis, Preciphar, Sandoz, Takeda, Thuasne, and UCB.
Dr. Lane had no relevant conflicts of interest to declare.
A version of this article appeared on Medscape.com.
VIENNA — Data do not back the use of diacerein or resveratrol for managing the pain of knee osteoarthritis (OA), according to the results of two well-performed, multicenter, double-blind, randomized controlled clinical trials.
During the News in Therapies session at the OARSI 2024 World Congress, the null findings of the DICKENS study and ARTHROL trial were presented alongside a reappraisal of the possible role of botulinum toxin.
DICKENS Study of Diacerein
“The role of diacerein in the treatment of OA is controversial,” acknowledged Dawn Aitken, PhD, associate professor at the University of Tasmania in Hobart, Tasmania, Australia. “There are only a few acceptable quality trials to date, and the results are inconsistent,” Dr. Aitken added.
Indeed, a Cochrane review performed in 2014 had concluded that there was “low-quality evidence that diacerein had a small beneficial effect on pain,” she said. The reported overall effect size on a 100-mm visual analog scale, based on a meta-analysis of 10 trials, has been just −8.65 mm, equating to just a 9% pain reduction.
At the time the DICKENS study was conceived, diacerein was recommended by a number of international guidelines for the management of hip and knee OA, although further, higher-quality studies were needed.
Diacerein blocks interleukin-1 beta, which is one of the key inflammatory markers of OA, so Dr. Aitken and collaborators postulated that perhaps it would work better if used in patients with an inflammatory phenotype.
They set about to test their hypothesis by recruiting 260 individuals with knee OA and MRI-detected effusion synovitis. The participants were then randomly allocated to treatment with either diacerein or a matching placebo for 24 weeks.
Individuals in the diacerein group were treated with an oral dose of 50 mg once daily for the first 2 weeks. If tolerated, the dose was increased to 50 mg twice daily.
No significant improvement in the primary endpoint of knee pain was seen comparing diacerein with placebo, with mean values of 53.2 mm and 56.4 mm, respectively, at 24 weeks using a 0-100 mm visual analog scale where 0 represented no pain and 100 represented the worst pain. It followed that there was no significant difference in the change from baseline to week 24 (−19.9 mm vs −18.6 mm; P = .77).
There was also no difference in the secondary endpoints, which included Western Ontario and McMaster Universities Arthritis Index pain, function, and stiffness. In fact, placebo-treated patients appeared to do better in terms of resolution of effusion synovitis as measured by a repeat MRI and quality of life, Dr. Aitken reported.
“These findings do not support the use of diacerein in treating patients with knee OA and effusion synovitis,” Dr. Aitken concluded.
ARTHROL Trial of Resveratrol
Similarly, negative results were reported for resveratrol from the ARTHROL trial, with 55% of the resveratrol- and 55% of placebo-treated individuals achieving a 20% reduction in knee pain intensity at 3 months. The actual change in knee pain from baseline to 3 months was −15.7 for resveratrol and −15.2 for placebo on a numerical rating scale that went from 0 (no pain) up to 100 (worst pain).
Resveratrol is found naturally in grapes, peanuts, pine cones, and Chinese knotweed, and there is a growing body of evidence that it may have pleiotropic effects, said investigator Christelle Nguyen, PhD, MD, a professor of physical and rehabilitation medicine at Université Paris Cité, Paris, France.
It’s available in a powder form over the counter as a treatment for multiple ailments, but more recently, became available as an oral formulation. Dr. Nguyen and colleagues wanted to know if this would make a difference to OA knee pain when added to usual care.
A double-blind, multicenter, placebo-controlled randomized trial was therefore conducted that involved 142 people with knee OA who had been experiencing knee pain for at least 1 month. The participants were equally randomly allocated to receive either oral resveratrol given as two caplets of 20 mg twice daily for the first week, then once daily for a total of 6 months, or a matched placebo.
There was also no effect of resveratrol vs placebo on a host of secondary outcomes measured at 3 and 6 months.
The interpretation is that oral resveratrol may not be effective in this indication or have a biologic effect on the pain pathway, Dr. Nguyen said.
“Our findings do not support the use of [trans-resveratrol] supplementation in this patented formulation for reducing knee pain in adults with painful knee OA,” she concluded.
Botulinum Toxin: Over But Not Out?
Dr. Nguyen separately reported data from a new systematic review and meta-analysis on the use of intra-articular (IA) botulinum toxin type A (BoNT-A) for knee OA pain.
Seven of the 14 randomized controlled trials included in the meta-analysis had looked specifically at knee OA outcomes in the short, intermediate, and long term.
Results showed a nonsignificant trend favoring BoNT-A use, with the standard mean difference in pain of 0.35 (−0.82; 0.12), −0.27 (−0.61; 0.08), and −0.43 (−1.12; 0.26) for short-, intermediate-, and long-term use, respectively.
In contrast, pain reductions were seen with BoNT-A in three trials that included people with OA of the shoulder or base of the thumb. This begs the question as to whether botulinum toxin may still have a role to play, Dr. Nguyen said in an interview.
“It seems like there may be a positive effect for the shoulder joint and base of the thumb,” she told this news organization.
“So, basically, we found differences between large and small to intermediate joints,” Dr. Nguyen added. “It questions the dilution of botulinum toxin into the joint. If it’s a big joint, maybe the dilution is too high,” she suggested.
This hypothesis will be tested in the upcoming RHIBOT II trial that will begin recruitment later this year. This is a follow-on from the RHIBOT trial that was published in The Lancet Rheumatology 2 years ago.
Meanwhile, the use of botulinum toxin is off-label, Dr. Nguyen said. “We use it in our clinics only when first-line treatment had failed for base of thumb OA.” It’s not offered as a stand-alone intervention, and the IA injections need to be given by someone with experience, she said.
Methodologically Sound Studies
Commenting on the studies, Nancy E. Lane, MD, said: “There have been small botulinum studies before but not powered enough so that you could confirm or refute hypotheses.”
Dr. Lane, endowed professor of medicine, rheumatology, and aging research and director for the Center for Musculoskeletal Health at the University of California Davis School of Medicine, Sacramento, California, added: “Similarly for resveratrol, there have been lots of studies.”
Moreover, Dr. Lane observed that the studies were “really well-designed. They were well-powered. The subjects were selected in such a way that was good rigor in the methodologic design, and there were enough people in the studies so that you could really believe the results.”
The take-home is probably that these approaches do not work, Dr. Lane said, “at least when you apply them to moderate-severe knee OA patients, they don’t seem to make a difference.”
The congress was sponsored by the Osteoarthritis Research Society International.
The DICKENS study of diacerein was an investigator-initiated trial that was funded by the National Health and Medical Research Council of Australia. TRB Chemedica International S.A. provided diacerein free of charge for the trial but was not involved in the implementation or data analysis. Dr. Aitken had no conflicts of interest to disclose.
The ARTHROL trial of oral resveratrol was funded by the French Ministry of Health and Solidarity (Ministré des Solidarités et de la Santé). Yvery Laboratory provided the resveratrol caplet and matching placebo free of charge. Dr. Nguyen has financial relationships with Actelion, Grünenthal, Ipsen, Lilly, Meda, Merz, Novartis, Preciphar, Sandoz, Takeda, Thuasne, and UCB.
Dr. Lane had no relevant conflicts of interest to declare.
A version of this article appeared on Medscape.com.
FROM OARSI 2024
Investing in the Future of GI
Talented young investigators are walking away from gastroenterology and hepatology research frustrated by a lack of support. For the last decades, Congress has slashed research funding and even greater cuts are on the horizon. Investigators in the early stages of their careers are particularly hard hit. Without help from other funding sources, young investigators struggle to continue their research, build their research portfolio, and obtain federal funding.
Decades of research have revolutionized the care of many digestive disease patients. These patients, as well as everyone in the gastroenterology and hepatology fields — clinicians and researchers alike — have benefited from the discoveries of dedicated investigators, past and present.
Right now, creative young researchers are poised to make groundbreaking discoveries that will shape the future of gastroenterology and hepatology. Unfortunately, declining government funding for biomedical research puts this potential in jeopardy. We’re at risk of losing an entire generation of researchers.
To fill this gap, the AGA Research Foundation invites you to support its mission by making a donation. Funds raised through the AGA Research Foundation will support the pipeline of new investigators’ research careers, allowing them to make discoveries that could ultimately improve patient care and even cure diseases.
“I donated to the AGA Research Foundation to ensure the vitality of our specialty, and to fund the research of future generations of gastroenterologists. Funding from organizations like the AGA Research Foundation is crucial for young scientists and gastroenterologists to launch their careers,” states Michael Camilleri, MD, AGAF, AGA Research Foundation Chair.
By joining others in supporting the AGA Research Foundation, you will ensure that young researchers have opportunities to continue their life-saving work. Learn more or make a contribution at www.foundation.gastro.org.
Talented young investigators are walking away from gastroenterology and hepatology research frustrated by a lack of support. For the last decades, Congress has slashed research funding and even greater cuts are on the horizon. Investigators in the early stages of their careers are particularly hard hit. Without help from other funding sources, young investigators struggle to continue their research, build their research portfolio, and obtain federal funding.
Decades of research have revolutionized the care of many digestive disease patients. These patients, as well as everyone in the gastroenterology and hepatology fields — clinicians and researchers alike — have benefited from the discoveries of dedicated investigators, past and present.
Right now, creative young researchers are poised to make groundbreaking discoveries that will shape the future of gastroenterology and hepatology. Unfortunately, declining government funding for biomedical research puts this potential in jeopardy. We’re at risk of losing an entire generation of researchers.
To fill this gap, the AGA Research Foundation invites you to support its mission by making a donation. Funds raised through the AGA Research Foundation will support the pipeline of new investigators’ research careers, allowing them to make discoveries that could ultimately improve patient care and even cure diseases.
“I donated to the AGA Research Foundation to ensure the vitality of our specialty, and to fund the research of future generations of gastroenterologists. Funding from organizations like the AGA Research Foundation is crucial for young scientists and gastroenterologists to launch their careers,” states Michael Camilleri, MD, AGAF, AGA Research Foundation Chair.
By joining others in supporting the AGA Research Foundation, you will ensure that young researchers have opportunities to continue their life-saving work. Learn more or make a contribution at www.foundation.gastro.org.
Talented young investigators are walking away from gastroenterology and hepatology research frustrated by a lack of support. For the last decades, Congress has slashed research funding and even greater cuts are on the horizon. Investigators in the early stages of their careers are particularly hard hit. Without help from other funding sources, young investigators struggle to continue their research, build their research portfolio, and obtain federal funding.
Decades of research have revolutionized the care of many digestive disease patients. These patients, as well as everyone in the gastroenterology and hepatology fields — clinicians and researchers alike — have benefited from the discoveries of dedicated investigators, past and present.
Right now, creative young researchers are poised to make groundbreaking discoveries that will shape the future of gastroenterology and hepatology. Unfortunately, declining government funding for biomedical research puts this potential in jeopardy. We’re at risk of losing an entire generation of researchers.
To fill this gap, the AGA Research Foundation invites you to support its mission by making a donation. Funds raised through the AGA Research Foundation will support the pipeline of new investigators’ research careers, allowing them to make discoveries that could ultimately improve patient care and even cure diseases.
“I donated to the AGA Research Foundation to ensure the vitality of our specialty, and to fund the research of future generations of gastroenterologists. Funding from organizations like the AGA Research Foundation is crucial for young scientists and gastroenterologists to launch their careers,” states Michael Camilleri, MD, AGAF, AGA Research Foundation Chair.
By joining others in supporting the AGA Research Foundation, you will ensure that young researchers have opportunities to continue their life-saving work. Learn more or make a contribution at www.foundation.gastro.org.