User login
Blood Biomarkers Predict Knee Osteoarthritis Years in Advance
A small number of blood biomarkers can identify patients who will develop knee osteoarthritis (OA) up to 8 years before signs of the disease are detectable via X-ray, according to new research.
The study “provides more evidence for a pre-radiographic phase of disease,” wrote Virginia Byers Dr. Kraus, MD, PhD, a professor of medicine, pathology, and orthopedic surgery at Duke University School of Medicine in Durham, North Carolina, and colleagues. The results also “provide valuable information for understanding the molecular events of early disease that could inform strategies to develop disease-modifying drugs for preclinical OA,” they continued.
In the study, published in Science Advances, researchers analyzed blood samples from a population-based, longitudinal study of women in London that assessed participants annually for osteoporosis and OA. They selected individuals at low risk for radiographic knee OA, who did not have traditional risk factors for knee OA such as a history of major knee injury, knee surgery, or OA of the hand or opposite knee.
The researchers analyzed serum of 100 women who went on to develop radiographic knee OA and 100 controls who were matched by age and body mass index (BMI). Participants were, on average, aged 54 years with a BMI of 26 and all were White. They analyzed serum peptides via mass spectrometry and used machine learning to select which out of the 115 identified peptides were most predictive of OA.
Ultimately, the team zeroed in on six peptides, corresponding to six proteins, that could most accurately distinguish women who went on to develop radiographic signs of OA from controls (area under the receiver operating characteristic curve, 0.77) up to 8 years before x-rays detected these changes.
“The value of our study is a panel that, in the absence of clinical factors indicative of high-risk knee OA, has the potential to discriminate individuals at risk for incident radiographic knee OA from those not at risk,” the authors wrote.
In earlier work, a similar group of biomarkers could accurately diagnose knee OA as well as predict the progression of the disease. More than half (58%) of biomarkers that predicted incident OA also predicted OA progression.
“Even for the ones that didn’t overlap with OA progression, they all pointed to the same sort of disease process, which is an unresolved acute phase response type of biological process,” Dr. Kraus told this news organization.
Commenting on the study, Andrew Grose, MD, an orthopedic trauma surgeon at the Hospital for Special Surgery in New York City, noted that the methods and conclusions seemed sound but cautioned that the study only looked for radiographic evidence of OA, and not symptomatic OA.
“Clinically relevant OA is correlated with what you see on an x-ray, but the x-ray is definitely not the whole story,” he said in an interview with this news organization.
To be clinically relevant, patients must also have symptoms, such as pain and stiffness, and interfere with daily life. But what shows up on an x-ray is not necessarily indicative of what patients are experiencing, he said. Solely focusing on radiographic findings could lead to overdiagnosis and overtreatment of OA, he said.
The study population was also small, and only included White women, he added, so further validation is necessary. Dr. Kraus and colleagues also acknowledged these limitations.
“Further validation will be needed in independent and larger cohorts, preferability prospectively collected and including male participants and the combination of incident radiographic and symptomatic OA,” they wrote. They noted that while this current study included only women, the biomarkers were not associated with sex in previous studies that used larger and mixed-sex cohorts.
“If they did more studies showing that this [test] was able to predict clinically relevant OA, then I think you could have a meaningful conversation with a patient in a primary care doctor’s office,” Dr. Grose added. “Until that time, just the fact that it predicts an x-ray finding is a little bit of a red herring.”
This work was supported by grants from the National Institutes of Health. Dr. Kraus is an inventor on a patent related to OA progression biomarkers. Dr. Grose had no relevant disclosures.
A version of this article appeared on Medscape.com.
A small number of blood biomarkers can identify patients who will develop knee osteoarthritis (OA) up to 8 years before signs of the disease are detectable via X-ray, according to new research.
The study “provides more evidence for a pre-radiographic phase of disease,” wrote Virginia Byers Dr. Kraus, MD, PhD, a professor of medicine, pathology, and orthopedic surgery at Duke University School of Medicine in Durham, North Carolina, and colleagues. The results also “provide valuable information for understanding the molecular events of early disease that could inform strategies to develop disease-modifying drugs for preclinical OA,” they continued.
In the study, published in Science Advances, researchers analyzed blood samples from a population-based, longitudinal study of women in London that assessed participants annually for osteoporosis and OA. They selected individuals at low risk for radiographic knee OA, who did not have traditional risk factors for knee OA such as a history of major knee injury, knee surgery, or OA of the hand or opposite knee.
The researchers analyzed serum of 100 women who went on to develop radiographic knee OA and 100 controls who were matched by age and body mass index (BMI). Participants were, on average, aged 54 years with a BMI of 26 and all were White. They analyzed serum peptides via mass spectrometry and used machine learning to select which out of the 115 identified peptides were most predictive of OA.
Ultimately, the team zeroed in on six peptides, corresponding to six proteins, that could most accurately distinguish women who went on to develop radiographic signs of OA from controls (area under the receiver operating characteristic curve, 0.77) up to 8 years before x-rays detected these changes.
“The value of our study is a panel that, in the absence of clinical factors indicative of high-risk knee OA, has the potential to discriminate individuals at risk for incident radiographic knee OA from those not at risk,” the authors wrote.
In earlier work, a similar group of biomarkers could accurately diagnose knee OA as well as predict the progression of the disease. More than half (58%) of biomarkers that predicted incident OA also predicted OA progression.
“Even for the ones that didn’t overlap with OA progression, they all pointed to the same sort of disease process, which is an unresolved acute phase response type of biological process,” Dr. Kraus told this news organization.
Commenting on the study, Andrew Grose, MD, an orthopedic trauma surgeon at the Hospital for Special Surgery in New York City, noted that the methods and conclusions seemed sound but cautioned that the study only looked for radiographic evidence of OA, and not symptomatic OA.
“Clinically relevant OA is correlated with what you see on an x-ray, but the x-ray is definitely not the whole story,” he said in an interview with this news organization.
To be clinically relevant, patients must also have symptoms, such as pain and stiffness, and interfere with daily life. But what shows up on an x-ray is not necessarily indicative of what patients are experiencing, he said. Solely focusing on radiographic findings could lead to overdiagnosis and overtreatment of OA, he said.
The study population was also small, and only included White women, he added, so further validation is necessary. Dr. Kraus and colleagues also acknowledged these limitations.
“Further validation will be needed in independent and larger cohorts, preferability prospectively collected and including male participants and the combination of incident radiographic and symptomatic OA,” they wrote. They noted that while this current study included only women, the biomarkers were not associated with sex in previous studies that used larger and mixed-sex cohorts.
“If they did more studies showing that this [test] was able to predict clinically relevant OA, then I think you could have a meaningful conversation with a patient in a primary care doctor’s office,” Dr. Grose added. “Until that time, just the fact that it predicts an x-ray finding is a little bit of a red herring.”
This work was supported by grants from the National Institutes of Health. Dr. Kraus is an inventor on a patent related to OA progression biomarkers. Dr. Grose had no relevant disclosures.
A version of this article appeared on Medscape.com.
A small number of blood biomarkers can identify patients who will develop knee osteoarthritis (OA) up to 8 years before signs of the disease are detectable via X-ray, according to new research.
The study “provides more evidence for a pre-radiographic phase of disease,” wrote Virginia Byers Dr. Kraus, MD, PhD, a professor of medicine, pathology, and orthopedic surgery at Duke University School of Medicine in Durham, North Carolina, and colleagues. The results also “provide valuable information for understanding the molecular events of early disease that could inform strategies to develop disease-modifying drugs for preclinical OA,” they continued.
In the study, published in Science Advances, researchers analyzed blood samples from a population-based, longitudinal study of women in London that assessed participants annually for osteoporosis and OA. They selected individuals at low risk for radiographic knee OA, who did not have traditional risk factors for knee OA such as a history of major knee injury, knee surgery, or OA of the hand or opposite knee.
The researchers analyzed serum of 100 women who went on to develop radiographic knee OA and 100 controls who were matched by age and body mass index (BMI). Participants were, on average, aged 54 years with a BMI of 26 and all were White. They analyzed serum peptides via mass spectrometry and used machine learning to select which out of the 115 identified peptides were most predictive of OA.
Ultimately, the team zeroed in on six peptides, corresponding to six proteins, that could most accurately distinguish women who went on to develop radiographic signs of OA from controls (area under the receiver operating characteristic curve, 0.77) up to 8 years before x-rays detected these changes.
“The value of our study is a panel that, in the absence of clinical factors indicative of high-risk knee OA, has the potential to discriminate individuals at risk for incident radiographic knee OA from those not at risk,” the authors wrote.
In earlier work, a similar group of biomarkers could accurately diagnose knee OA as well as predict the progression of the disease. More than half (58%) of biomarkers that predicted incident OA also predicted OA progression.
“Even for the ones that didn’t overlap with OA progression, they all pointed to the same sort of disease process, which is an unresolved acute phase response type of biological process,” Dr. Kraus told this news organization.
Commenting on the study, Andrew Grose, MD, an orthopedic trauma surgeon at the Hospital for Special Surgery in New York City, noted that the methods and conclusions seemed sound but cautioned that the study only looked for radiographic evidence of OA, and not symptomatic OA.
“Clinically relevant OA is correlated with what you see on an x-ray, but the x-ray is definitely not the whole story,” he said in an interview with this news organization.
To be clinically relevant, patients must also have symptoms, such as pain and stiffness, and interfere with daily life. But what shows up on an x-ray is not necessarily indicative of what patients are experiencing, he said. Solely focusing on radiographic findings could lead to overdiagnosis and overtreatment of OA, he said.
The study population was also small, and only included White women, he added, so further validation is necessary. Dr. Kraus and colleagues also acknowledged these limitations.
“Further validation will be needed in independent and larger cohorts, preferability prospectively collected and including male participants and the combination of incident radiographic and symptomatic OA,” they wrote. They noted that while this current study included only women, the biomarkers were not associated with sex in previous studies that used larger and mixed-sex cohorts.
“If they did more studies showing that this [test] was able to predict clinically relevant OA, then I think you could have a meaningful conversation with a patient in a primary care doctor’s office,” Dr. Grose added. “Until that time, just the fact that it predicts an x-ray finding is a little bit of a red herring.”
This work was supported by grants from the National Institutes of Health. Dr. Kraus is an inventor on a patent related to OA progression biomarkers. Dr. Grose had no relevant disclosures.
A version of this article appeared on Medscape.com.
FROM SCIENCE ADVANCES
Avian Flu Threat Still Low and Vaccine Measures Are Ready
After cow-to-cow transmission of avian influenza A subtype H5N1 in US dairy herds led to a cow-to-human transmission in Texas, the Association of State and Territorial Health Officials convened a panel of experts for a scientific symposium on Thursday to talk about the public health implications.
From the sequencing data, “we can expect and anticipate that [the candidate vaccine viruses] will provide good protection,” she explained.
Establishing candidate vaccine viruses “are the precursor to moving into large-scale vaccine production,” Dr. Dugan explained. Should that be needed, the candidate viruses can be used by manufacturers to produce new vaccines.
The CDC is also actively partnering with commercial diagnostic developers and testing companies in case there is a need to scale-up testing, Dr. Dugan said.
The only current human case in the United States was reported on April 1 and confirmed by the CDC within 24 hours, reported Sonja Olsen, PhD, associate director for preparedness and response of the Influenza Division at the CDC.
The person had direct exposure to cattle and reported eye redness, consistent with conjunctivitis, as the only symptom. The person received treatment and has recovered, and there were no reports of illness among the person’s household contacts, Dr. Olsen said.
Person With the Virus Has Recovered
The only other detection of the virus in a human in the United States was in 2022 and it was associated with infected poultry exposure. That person also had mild illness and recovered, Dr. Olsen explained.
Since 1997, when the first case of human infection was reported globally, “there have been 909 [human cases] reported from 23 countries,” Dr. Olsen said. “About half [52%] of the human cases have resulted in death.” Only a small number of human cases have been reported since 2015, but since 2022, more than two dozen human cases have been reported to the World Health Organization.
Experience with the virus in the United States has been about a year behind that in Europe, said Rosemary Sifford, DVM, chief veterinary officer at the US Department of Agriculture. In the United States, the first detection — in January 2022 — was in wild birds; this was followed the next month by the first detection in a commercial poultry flock.
In March of this year, the United States had its first detection in cattle, specifically dairy cattle. But testing has shown that “it remains very much an avian virus. It’s not becoming a bovine virus,” Dr. Sifford reported.
Detected in Cattle
Earlier this week, in an effort to minimize the risk of disease spread, the USDA issued a federal order that requires the reporting of positive influenza tests in livestock and mandatory testing for influenza of dairy cattle before interstate movement.
“As of today, there are affected herds in 33 farms across eight states,” reported Dr. Olsen.
Tests are ongoing to determine how the virus is traveling, but “what we can say is that there’s a high viral load in the milk in the cattle, and it appears that the transmission is happening mostly within the lactating herds,” Dr. Sifford reported. It is unclear whether that is happening during the milking of the cows or whether contaminated milk from a cow with a high viral load is transmitting the virus to other cattle.
“We are strongly encouraging producers to limit the movement of cattle, particularly lactating cattle, as much as possible,” she says.
Milk Is Likely the Source of Transmission
“We haven’t seen anything that would change our assessment that the commercial milk supply is safe,” says Donald Prater, DVM, acting director of the Center for Food Safety and Applied Nutrition at the US Food and Drug Administration (FDA).
In the federal and state milk safety system, he explained, nearly 99% of the commercial milk supply comes from farms that participate in the Grade A program and follow the Pasteurized Milk Ordinance, which outlines pasteurization requirements.
Because detection of the virus in dairy cattle is new, there are many questions to be answered in research, he reported. Among them:
- What level of virus might be leaving the farms from shedding by apparently healthy cows?
- Does any live virus survive the pasteurization process?
- Do different methods of pasteurization and dairy production have different effects on the viability of H5N1?
- Are effects different in various forms of dairy products, such as cheese and cream?
A critical question regarding the potential risk to humans is how much milk would have to be consumed for the virus to become an established infection. That information is essential to determine “what type of pasteurization criteria” are needed to provide “acceptable public health outcomes,” Dr. Prater said.
The CDC is currently using the flu surveillance system to monitor for H5N1 activity in people. The systems show no current indicators of unusual influenza activity in people.
A version of this article appeared on Medscape.com.
After cow-to-cow transmission of avian influenza A subtype H5N1 in US dairy herds led to a cow-to-human transmission in Texas, the Association of State and Territorial Health Officials convened a panel of experts for a scientific symposium on Thursday to talk about the public health implications.
From the sequencing data, “we can expect and anticipate that [the candidate vaccine viruses] will provide good protection,” she explained.
Establishing candidate vaccine viruses “are the precursor to moving into large-scale vaccine production,” Dr. Dugan explained. Should that be needed, the candidate viruses can be used by manufacturers to produce new vaccines.
The CDC is also actively partnering with commercial diagnostic developers and testing companies in case there is a need to scale-up testing, Dr. Dugan said.
The only current human case in the United States was reported on April 1 and confirmed by the CDC within 24 hours, reported Sonja Olsen, PhD, associate director for preparedness and response of the Influenza Division at the CDC.
The person had direct exposure to cattle and reported eye redness, consistent with conjunctivitis, as the only symptom. The person received treatment and has recovered, and there were no reports of illness among the person’s household contacts, Dr. Olsen said.
Person With the Virus Has Recovered
The only other detection of the virus in a human in the United States was in 2022 and it was associated with infected poultry exposure. That person also had mild illness and recovered, Dr. Olsen explained.
Since 1997, when the first case of human infection was reported globally, “there have been 909 [human cases] reported from 23 countries,” Dr. Olsen said. “About half [52%] of the human cases have resulted in death.” Only a small number of human cases have been reported since 2015, but since 2022, more than two dozen human cases have been reported to the World Health Organization.
Experience with the virus in the United States has been about a year behind that in Europe, said Rosemary Sifford, DVM, chief veterinary officer at the US Department of Agriculture. In the United States, the first detection — in January 2022 — was in wild birds; this was followed the next month by the first detection in a commercial poultry flock.
In March of this year, the United States had its first detection in cattle, specifically dairy cattle. But testing has shown that “it remains very much an avian virus. It’s not becoming a bovine virus,” Dr. Sifford reported.
Detected in Cattle
Earlier this week, in an effort to minimize the risk of disease spread, the USDA issued a federal order that requires the reporting of positive influenza tests in livestock and mandatory testing for influenza of dairy cattle before interstate movement.
“As of today, there are affected herds in 33 farms across eight states,” reported Dr. Olsen.
Tests are ongoing to determine how the virus is traveling, but “what we can say is that there’s a high viral load in the milk in the cattle, and it appears that the transmission is happening mostly within the lactating herds,” Dr. Sifford reported. It is unclear whether that is happening during the milking of the cows or whether contaminated milk from a cow with a high viral load is transmitting the virus to other cattle.
“We are strongly encouraging producers to limit the movement of cattle, particularly lactating cattle, as much as possible,” she says.
Milk Is Likely the Source of Transmission
“We haven’t seen anything that would change our assessment that the commercial milk supply is safe,” says Donald Prater, DVM, acting director of the Center for Food Safety and Applied Nutrition at the US Food and Drug Administration (FDA).
In the federal and state milk safety system, he explained, nearly 99% of the commercial milk supply comes from farms that participate in the Grade A program and follow the Pasteurized Milk Ordinance, which outlines pasteurization requirements.
Because detection of the virus in dairy cattle is new, there are many questions to be answered in research, he reported. Among them:
- What level of virus might be leaving the farms from shedding by apparently healthy cows?
- Does any live virus survive the pasteurization process?
- Do different methods of pasteurization and dairy production have different effects on the viability of H5N1?
- Are effects different in various forms of dairy products, such as cheese and cream?
A critical question regarding the potential risk to humans is how much milk would have to be consumed for the virus to become an established infection. That information is essential to determine “what type of pasteurization criteria” are needed to provide “acceptable public health outcomes,” Dr. Prater said.
The CDC is currently using the flu surveillance system to monitor for H5N1 activity in people. The systems show no current indicators of unusual influenza activity in people.
A version of this article appeared on Medscape.com.
After cow-to-cow transmission of avian influenza A subtype H5N1 in US dairy herds led to a cow-to-human transmission in Texas, the Association of State and Territorial Health Officials convened a panel of experts for a scientific symposium on Thursday to talk about the public health implications.
From the sequencing data, “we can expect and anticipate that [the candidate vaccine viruses] will provide good protection,” she explained.
Establishing candidate vaccine viruses “are the precursor to moving into large-scale vaccine production,” Dr. Dugan explained. Should that be needed, the candidate viruses can be used by manufacturers to produce new vaccines.
The CDC is also actively partnering with commercial diagnostic developers and testing companies in case there is a need to scale-up testing, Dr. Dugan said.
The only current human case in the United States was reported on April 1 and confirmed by the CDC within 24 hours, reported Sonja Olsen, PhD, associate director for preparedness and response of the Influenza Division at the CDC.
The person had direct exposure to cattle and reported eye redness, consistent with conjunctivitis, as the only symptom. The person received treatment and has recovered, and there were no reports of illness among the person’s household contacts, Dr. Olsen said.
Person With the Virus Has Recovered
The only other detection of the virus in a human in the United States was in 2022 and it was associated with infected poultry exposure. That person also had mild illness and recovered, Dr. Olsen explained.
Since 1997, when the first case of human infection was reported globally, “there have been 909 [human cases] reported from 23 countries,” Dr. Olsen said. “About half [52%] of the human cases have resulted in death.” Only a small number of human cases have been reported since 2015, but since 2022, more than two dozen human cases have been reported to the World Health Organization.
Experience with the virus in the United States has been about a year behind that in Europe, said Rosemary Sifford, DVM, chief veterinary officer at the US Department of Agriculture. In the United States, the first detection — in January 2022 — was in wild birds; this was followed the next month by the first detection in a commercial poultry flock.
In March of this year, the United States had its first detection in cattle, specifically dairy cattle. But testing has shown that “it remains very much an avian virus. It’s not becoming a bovine virus,” Dr. Sifford reported.
Detected in Cattle
Earlier this week, in an effort to minimize the risk of disease spread, the USDA issued a federal order that requires the reporting of positive influenza tests in livestock and mandatory testing for influenza of dairy cattle before interstate movement.
“As of today, there are affected herds in 33 farms across eight states,” reported Dr. Olsen.
Tests are ongoing to determine how the virus is traveling, but “what we can say is that there’s a high viral load in the milk in the cattle, and it appears that the transmission is happening mostly within the lactating herds,” Dr. Sifford reported. It is unclear whether that is happening during the milking of the cows or whether contaminated milk from a cow with a high viral load is transmitting the virus to other cattle.
“We are strongly encouraging producers to limit the movement of cattle, particularly lactating cattle, as much as possible,” she says.
Milk Is Likely the Source of Transmission
“We haven’t seen anything that would change our assessment that the commercial milk supply is safe,” says Donald Prater, DVM, acting director of the Center for Food Safety and Applied Nutrition at the US Food and Drug Administration (FDA).
In the federal and state milk safety system, he explained, nearly 99% of the commercial milk supply comes from farms that participate in the Grade A program and follow the Pasteurized Milk Ordinance, which outlines pasteurization requirements.
Because detection of the virus in dairy cattle is new, there are many questions to be answered in research, he reported. Among them:
- What level of virus might be leaving the farms from shedding by apparently healthy cows?
- Does any live virus survive the pasteurization process?
- Do different methods of pasteurization and dairy production have different effects on the viability of H5N1?
- Are effects different in various forms of dairy products, such as cheese and cream?
A critical question regarding the potential risk to humans is how much milk would have to be consumed for the virus to become an established infection. That information is essential to determine “what type of pasteurization criteria” are needed to provide “acceptable public health outcomes,” Dr. Prater said.
The CDC is currently using the flu surveillance system to monitor for H5N1 activity in people. The systems show no current indicators of unusual influenza activity in people.
A version of this article appeared on Medscape.com.
Working Hard or Work Addiction — Have You Crossed the Line?
When child psychiatrist Javeed Sukhera, MD, PhD, was a few years into his career, he found himself doing it all. “I was in a leadership role academically at the medical school, I had a leadership role at the hospital, and I was seeing as many patients as I could. I could work all day every day.”
“It still wouldn’t have been enough,” he said.
Whenever there was a shift available, Dr. Sukhera would take it. His job was stressful, but as a new physician with a young family, he saw this obsession with work as necessary. “I began to cope with the stress from work by doing extra work and feeling like I needed to be everywhere. It was like I became a hamster on a spinning wheel. I was just running, running, running.”
Things shifted for Dr. Sukhera when he realized that while he was emotionally available for the children who were his patients, at home, his own children weren’t getting the best of him. “There was a specific moment when I thought my son was afraid of me,” he said. “I just stopped and realized that there was something happening that I needed to break. I needed to make a change.”
Dr. Sukhera, now chair of psychiatry at the Institute of Living and chief of the Department of Psychiatry at Hartford Hospital, Hartford, Connecticut, believes what he experienced was a steep fall into work addiction.
What Does Work Addiction Look Like for Doctors?
Behavioral addictions are fairly new in the addiction space. When gambling disorder, the first and only behavioral addiction in the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, was added in 2013, it was seen as a “breakthrough addiction,” said Mark D. Griffiths, PhD, a leading behavioral addiction researcher and a distinguished professor at Nottingham Trent University.
Because there is not enough evidence yet to classify work addiction as a formal diagnosis, there is no clear consensus on how to define it. To further complicate things, the terms “workaholism” and “work addiction” can be used interchangeably, and some experts say the two are not the same, though they can overlap.
That said, a 2018 review of literature from several countries found that work addiction “fits very well into recently postulated criteria for conceptualization of a behavioral addiction.
“If you accept that gambling can be genuinely addictive, then there’s no reason to think that something like work, exercise, or video game playing couldn’t be an addiction as well,” said Dr. Griffiths.
“The neurobiology of addiction is that we get drawn to something that gives us a dopamine hit,” Dr. Sukhera added. “But to do that all day, every day, has consequences. It drains our emotional reserves, and it can greatly impact our relationships.”
On top of that, work addiction has been linked with poor sleep, poor cardiovascular health, high blood pressure, burnout, the development of autoimmune disorders, and other health issues.
Physicians are particularly susceptible. Doctors, after all, are expected to work long hours and put their patients’ needs first, even at the expense of their own health and well-being.
“Workaholism is not just socially acceptable in medicine,” said Dr. Sukhera. “It’s baked into the system and built into the structures. The healthcare system has largely functioned on the emotional labor of health workers, whose tendency to show up and work harder can, at times, in certain organizations, be exploited.”
Dr. Griffiths agreed that with the limited amount of data available, work addiction does appear to exist at higher rates in medicine than in other fields. As early as the 1970s, medical literature describes work as a “socially acceptable” addiction among doctors. A 2014 study published in Occupational Medicine reported that of 445 physicians who took part in the research, nearly half exhibited some level of work addiction with 13% “highly work addicted.”
Of course, working hard or even meeting unreasonable demands from work is not the same as work addiction, as Dr. Griffiths clarified in a 2023 editorial in BMJ Quality & Safety. The difference, as with other behavioral addictions, is when people obsess about work and use it to cope with stress. It can be easier to stay distracted and busy to gain a sense of control rather than learning to deal with complex emotions.
A 2021 study that Dr. Sukhera conducted with resident physicians found that working harder was one of the main ways they dealt with stress during the COVID-19 pandemic. “This idea that we deal with the stress of being burnt out by doing more and more of what burns us out is fairly ubiquitous at all stages of medical professionals’ careers,” he said.
Financial incentives also can fuel work addiction, said Dr. Sukhera. In residency, there are some safeguards around overwork and duty hours. When you become an attending, those limits no longer exist. As a young physician, Dr. Sukhera had student debt to pay off and a family to support. When he found opportunities to earn more by working more, his answer was always “yes.”
Pressure to produce medical research also can pose issues. Some physicians can become addicted to publishing studies, fearing that they might lose their professional status or position if they stop. It’s a cycle that can force a doctor to not only work long hours doing their job but also practically take on a second one.
How Physicians Can Recognize Work Addiction in Themselves
Work addiction can look and feel different for every person, said Malissa Clark, PhD, associate professor at the University of Georgia and author of the recent book Never Not Working: Why the Always-On Culture Is Bad for Business—and How to Fix It.
Dr. Clark noted that people who are highly engaged in their work tend to be driven by intrinsic motivation: “You work because you love it.” With work addiction, “you work because you feel like you ought to be working all the time.”
Of course, it’s not always so cut and dried; you can experience both forms of motivation and not necessarily become addicted to work. But if you are solely driven by the feeling that you ought to be working all the time, that can be a red flag.
Dr. Griffiths said that while many people may have problematic work habits or work too much, true work addicts must meet six criteria that apply to all addictions:
1. Salience: Work is the single most important thing in your life, to the point of neglecting everything else. Even if you’re on vacation, your mind might be flooded with work thoughts.
2. Mood modification: You use work to modify your mood, either to get a “high” or to cope with stress.
3. Tolerance: Over time, you’ve gone from working 8 or 10 hours a day to 12 hours a day, to a point where you’re working all the time.
4. Withdrawal: On a physiological level, you will have symptoms such as anxiety, nausea, or headaches when unable to work.
5. Conflict: You feel conflicted with yourself (you know you’re working too much) or with others (partners, friends, and children) about work, but you can’t stop.
6. Relapse: If you manage to cut down your hours but can’t resist overworking 1 day, you wind up right back where you were.
When It’s Time to Address Work Addiction
The lack of a formal diagnosis for work addiction makes getting treatment difficult. But there are ways to seek help. Unlike the drug and alcohol literature, abstinence is not the goal. “The therapeutic goal is getting a behavior under control and looking for the triggers of why you’re compulsively working,” said Dr. Griffiths.
Practice self-compassion
Dr. Sukhera eventually realized that his work addiction stemmed from the fear of being somehow excluded or unworthy. He actively corrected much of this through self-compassion and self-kindness, which helped him set boundaries. “Self-compassion is the root of everything,” he said. “Reminding ourselves that we’re doing our best is an important ingredient in breaking the cycle.”
Slowly expose yourself to relaxation
Many workaholics find rest very difficult. “When I conducted interviews with people [who considered themselves workaholics], a very common thing I heard was, ‘I have a very hard time being idle,’ ” said Dr. Clark. If rest feels hard, Dr. Sukhera suggests practicing relaxation for 2 minutes to start. Even small periods of downtime can challenge the belief that you must be constantly productive.
Reframe your to-do list
For work addicts, to-do lists can seem like they must be finished, which prolongs work hours. Instead, use to-do lists to help prioritize what is urgent, identify what can wait, and delegate out tasks to others, Dr. Clark recommends.
Pick up a mastery experience
Research from professor Sabine Sonnentag, Dr. rer. nat., at the University of Mannheim, Mannheim, Germany, suggests that mastery experiences — leisure activities that require thought and focus like learning a new language or taking a woodworking class — can help you actively disengage from work.
Try cognitive behavioral therapy
Widely used for other forms of addiction, cognitive behavioral therapy centers around recognizing emotions, challenging thought patterns, and changing behaviors. However, Dr. Clark admits the research on its impact on work addiction, in particular, is “pretty nascent.”
Shift your mindset
It seems logical to think that detaching from your feelings will allow you to “do more,” but experts say that idea is both untrue and dangerous. “The safest hospitals are the hospitals where people are attuned to their humanness,” said Dr. Sukhera. “It’s normal to overwork in medicine, and if you’re challenging a norm, you really have to be thoughtful about how you frame that for yourself.”
Most importantly: Seek support
Today, there is increased awareness about work addiction and more resources for physicians who are struggling, including programs such as Workaholics Anonymous or Physicians Anonymous and workplace wellness initiatives. But try not to overwhelm yourself with choosing whom to talk to or what specific resource to utilize, Dr. Sukhera advised. “Just talk to someone about it. You don’t have to carry this on your own.”
A version of this article appeared on Medscape.com.
When child psychiatrist Javeed Sukhera, MD, PhD, was a few years into his career, he found himself doing it all. “I was in a leadership role academically at the medical school, I had a leadership role at the hospital, and I was seeing as many patients as I could. I could work all day every day.”
“It still wouldn’t have been enough,” he said.
Whenever there was a shift available, Dr. Sukhera would take it. His job was stressful, but as a new physician with a young family, he saw this obsession with work as necessary. “I began to cope with the stress from work by doing extra work and feeling like I needed to be everywhere. It was like I became a hamster on a spinning wheel. I was just running, running, running.”
Things shifted for Dr. Sukhera when he realized that while he was emotionally available for the children who were his patients, at home, his own children weren’t getting the best of him. “There was a specific moment when I thought my son was afraid of me,” he said. “I just stopped and realized that there was something happening that I needed to break. I needed to make a change.”
Dr. Sukhera, now chair of psychiatry at the Institute of Living and chief of the Department of Psychiatry at Hartford Hospital, Hartford, Connecticut, believes what he experienced was a steep fall into work addiction.
What Does Work Addiction Look Like for Doctors?
Behavioral addictions are fairly new in the addiction space. When gambling disorder, the first and only behavioral addiction in the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, was added in 2013, it was seen as a “breakthrough addiction,” said Mark D. Griffiths, PhD, a leading behavioral addiction researcher and a distinguished professor at Nottingham Trent University.
Because there is not enough evidence yet to classify work addiction as a formal diagnosis, there is no clear consensus on how to define it. To further complicate things, the terms “workaholism” and “work addiction” can be used interchangeably, and some experts say the two are not the same, though they can overlap.
That said, a 2018 review of literature from several countries found that work addiction “fits very well into recently postulated criteria for conceptualization of a behavioral addiction.
“If you accept that gambling can be genuinely addictive, then there’s no reason to think that something like work, exercise, or video game playing couldn’t be an addiction as well,” said Dr. Griffiths.
“The neurobiology of addiction is that we get drawn to something that gives us a dopamine hit,” Dr. Sukhera added. “But to do that all day, every day, has consequences. It drains our emotional reserves, and it can greatly impact our relationships.”
On top of that, work addiction has been linked with poor sleep, poor cardiovascular health, high blood pressure, burnout, the development of autoimmune disorders, and other health issues.
Physicians are particularly susceptible. Doctors, after all, are expected to work long hours and put their patients’ needs first, even at the expense of their own health and well-being.
“Workaholism is not just socially acceptable in medicine,” said Dr. Sukhera. “It’s baked into the system and built into the structures. The healthcare system has largely functioned on the emotional labor of health workers, whose tendency to show up and work harder can, at times, in certain organizations, be exploited.”
Dr. Griffiths agreed that with the limited amount of data available, work addiction does appear to exist at higher rates in medicine than in other fields. As early as the 1970s, medical literature describes work as a “socially acceptable” addiction among doctors. A 2014 study published in Occupational Medicine reported that of 445 physicians who took part in the research, nearly half exhibited some level of work addiction with 13% “highly work addicted.”
Of course, working hard or even meeting unreasonable demands from work is not the same as work addiction, as Dr. Griffiths clarified in a 2023 editorial in BMJ Quality & Safety. The difference, as with other behavioral addictions, is when people obsess about work and use it to cope with stress. It can be easier to stay distracted and busy to gain a sense of control rather than learning to deal with complex emotions.
A 2021 study that Dr. Sukhera conducted with resident physicians found that working harder was one of the main ways they dealt with stress during the COVID-19 pandemic. “This idea that we deal with the stress of being burnt out by doing more and more of what burns us out is fairly ubiquitous at all stages of medical professionals’ careers,” he said.
Financial incentives also can fuel work addiction, said Dr. Sukhera. In residency, there are some safeguards around overwork and duty hours. When you become an attending, those limits no longer exist. As a young physician, Dr. Sukhera had student debt to pay off and a family to support. When he found opportunities to earn more by working more, his answer was always “yes.”
Pressure to produce medical research also can pose issues. Some physicians can become addicted to publishing studies, fearing that they might lose their professional status or position if they stop. It’s a cycle that can force a doctor to not only work long hours doing their job but also practically take on a second one.
How Physicians Can Recognize Work Addiction in Themselves
Work addiction can look and feel different for every person, said Malissa Clark, PhD, associate professor at the University of Georgia and author of the recent book Never Not Working: Why the Always-On Culture Is Bad for Business—and How to Fix It.
Dr. Clark noted that people who are highly engaged in their work tend to be driven by intrinsic motivation: “You work because you love it.” With work addiction, “you work because you feel like you ought to be working all the time.”
Of course, it’s not always so cut and dried; you can experience both forms of motivation and not necessarily become addicted to work. But if you are solely driven by the feeling that you ought to be working all the time, that can be a red flag.
Dr. Griffiths said that while many people may have problematic work habits or work too much, true work addicts must meet six criteria that apply to all addictions:
1. Salience: Work is the single most important thing in your life, to the point of neglecting everything else. Even if you’re on vacation, your mind might be flooded with work thoughts.
2. Mood modification: You use work to modify your mood, either to get a “high” or to cope with stress.
3. Tolerance: Over time, you’ve gone from working 8 or 10 hours a day to 12 hours a day, to a point where you’re working all the time.
4. Withdrawal: On a physiological level, you will have symptoms such as anxiety, nausea, or headaches when unable to work.
5. Conflict: You feel conflicted with yourself (you know you’re working too much) or with others (partners, friends, and children) about work, but you can’t stop.
6. Relapse: If you manage to cut down your hours but can’t resist overworking 1 day, you wind up right back where you were.
When It’s Time to Address Work Addiction
The lack of a formal diagnosis for work addiction makes getting treatment difficult. But there are ways to seek help. Unlike the drug and alcohol literature, abstinence is not the goal. “The therapeutic goal is getting a behavior under control and looking for the triggers of why you’re compulsively working,” said Dr. Griffiths.
Practice self-compassion
Dr. Sukhera eventually realized that his work addiction stemmed from the fear of being somehow excluded or unworthy. He actively corrected much of this through self-compassion and self-kindness, which helped him set boundaries. “Self-compassion is the root of everything,” he said. “Reminding ourselves that we’re doing our best is an important ingredient in breaking the cycle.”
Slowly expose yourself to relaxation
Many workaholics find rest very difficult. “When I conducted interviews with people [who considered themselves workaholics], a very common thing I heard was, ‘I have a very hard time being idle,’ ” said Dr. Clark. If rest feels hard, Dr. Sukhera suggests practicing relaxation for 2 minutes to start. Even small periods of downtime can challenge the belief that you must be constantly productive.
Reframe your to-do list
For work addicts, to-do lists can seem like they must be finished, which prolongs work hours. Instead, use to-do lists to help prioritize what is urgent, identify what can wait, and delegate out tasks to others, Dr. Clark recommends.
Pick up a mastery experience
Research from professor Sabine Sonnentag, Dr. rer. nat., at the University of Mannheim, Mannheim, Germany, suggests that mastery experiences — leisure activities that require thought and focus like learning a new language or taking a woodworking class — can help you actively disengage from work.
Try cognitive behavioral therapy
Widely used for other forms of addiction, cognitive behavioral therapy centers around recognizing emotions, challenging thought patterns, and changing behaviors. However, Dr. Clark admits the research on its impact on work addiction, in particular, is “pretty nascent.”
Shift your mindset
It seems logical to think that detaching from your feelings will allow you to “do more,” but experts say that idea is both untrue and dangerous. “The safest hospitals are the hospitals where people are attuned to their humanness,” said Dr. Sukhera. “It’s normal to overwork in medicine, and if you’re challenging a norm, you really have to be thoughtful about how you frame that for yourself.”
Most importantly: Seek support
Today, there is increased awareness about work addiction and more resources for physicians who are struggling, including programs such as Workaholics Anonymous or Physicians Anonymous and workplace wellness initiatives. But try not to overwhelm yourself with choosing whom to talk to or what specific resource to utilize, Dr. Sukhera advised. “Just talk to someone about it. You don’t have to carry this on your own.”
A version of this article appeared on Medscape.com.
When child psychiatrist Javeed Sukhera, MD, PhD, was a few years into his career, he found himself doing it all. “I was in a leadership role academically at the medical school, I had a leadership role at the hospital, and I was seeing as many patients as I could. I could work all day every day.”
“It still wouldn’t have been enough,” he said.
Whenever there was a shift available, Dr. Sukhera would take it. His job was stressful, but as a new physician with a young family, he saw this obsession with work as necessary. “I began to cope with the stress from work by doing extra work and feeling like I needed to be everywhere. It was like I became a hamster on a spinning wheel. I was just running, running, running.”
Things shifted for Dr. Sukhera when he realized that while he was emotionally available for the children who were his patients, at home, his own children weren’t getting the best of him. “There was a specific moment when I thought my son was afraid of me,” he said. “I just stopped and realized that there was something happening that I needed to break. I needed to make a change.”
Dr. Sukhera, now chair of psychiatry at the Institute of Living and chief of the Department of Psychiatry at Hartford Hospital, Hartford, Connecticut, believes what he experienced was a steep fall into work addiction.
What Does Work Addiction Look Like for Doctors?
Behavioral addictions are fairly new in the addiction space. When gambling disorder, the first and only behavioral addiction in the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, was added in 2013, it was seen as a “breakthrough addiction,” said Mark D. Griffiths, PhD, a leading behavioral addiction researcher and a distinguished professor at Nottingham Trent University.
Because there is not enough evidence yet to classify work addiction as a formal diagnosis, there is no clear consensus on how to define it. To further complicate things, the terms “workaholism” and “work addiction” can be used interchangeably, and some experts say the two are not the same, though they can overlap.
That said, a 2018 review of literature from several countries found that work addiction “fits very well into recently postulated criteria for conceptualization of a behavioral addiction.
“If you accept that gambling can be genuinely addictive, then there’s no reason to think that something like work, exercise, or video game playing couldn’t be an addiction as well,” said Dr. Griffiths.
“The neurobiology of addiction is that we get drawn to something that gives us a dopamine hit,” Dr. Sukhera added. “But to do that all day, every day, has consequences. It drains our emotional reserves, and it can greatly impact our relationships.”
On top of that, work addiction has been linked with poor sleep, poor cardiovascular health, high blood pressure, burnout, the development of autoimmune disorders, and other health issues.
Physicians are particularly susceptible. Doctors, after all, are expected to work long hours and put their patients’ needs first, even at the expense of their own health and well-being.
“Workaholism is not just socially acceptable in medicine,” said Dr. Sukhera. “It’s baked into the system and built into the structures. The healthcare system has largely functioned on the emotional labor of health workers, whose tendency to show up and work harder can, at times, in certain organizations, be exploited.”
Dr. Griffiths agreed that with the limited amount of data available, work addiction does appear to exist at higher rates in medicine than in other fields. As early as the 1970s, medical literature describes work as a “socially acceptable” addiction among doctors. A 2014 study published in Occupational Medicine reported that of 445 physicians who took part in the research, nearly half exhibited some level of work addiction with 13% “highly work addicted.”
Of course, working hard or even meeting unreasonable demands from work is not the same as work addiction, as Dr. Griffiths clarified in a 2023 editorial in BMJ Quality & Safety. The difference, as with other behavioral addictions, is when people obsess about work and use it to cope with stress. It can be easier to stay distracted and busy to gain a sense of control rather than learning to deal with complex emotions.
A 2021 study that Dr. Sukhera conducted with resident physicians found that working harder was one of the main ways they dealt with stress during the COVID-19 pandemic. “This idea that we deal with the stress of being burnt out by doing more and more of what burns us out is fairly ubiquitous at all stages of medical professionals’ careers,” he said.
Financial incentives also can fuel work addiction, said Dr. Sukhera. In residency, there are some safeguards around overwork and duty hours. When you become an attending, those limits no longer exist. As a young physician, Dr. Sukhera had student debt to pay off and a family to support. When he found opportunities to earn more by working more, his answer was always “yes.”
Pressure to produce medical research also can pose issues. Some physicians can become addicted to publishing studies, fearing that they might lose their professional status or position if they stop. It’s a cycle that can force a doctor to not only work long hours doing their job but also practically take on a second one.
How Physicians Can Recognize Work Addiction in Themselves
Work addiction can look and feel different for every person, said Malissa Clark, PhD, associate professor at the University of Georgia and author of the recent book Never Not Working: Why the Always-On Culture Is Bad for Business—and How to Fix It.
Dr. Clark noted that people who are highly engaged in their work tend to be driven by intrinsic motivation: “You work because you love it.” With work addiction, “you work because you feel like you ought to be working all the time.”
Of course, it’s not always so cut and dried; you can experience both forms of motivation and not necessarily become addicted to work. But if you are solely driven by the feeling that you ought to be working all the time, that can be a red flag.
Dr. Griffiths said that while many people may have problematic work habits or work too much, true work addicts must meet six criteria that apply to all addictions:
1. Salience: Work is the single most important thing in your life, to the point of neglecting everything else. Even if you’re on vacation, your mind might be flooded with work thoughts.
2. Mood modification: You use work to modify your mood, either to get a “high” or to cope with stress.
3. Tolerance: Over time, you’ve gone from working 8 or 10 hours a day to 12 hours a day, to a point where you’re working all the time.
4. Withdrawal: On a physiological level, you will have symptoms such as anxiety, nausea, or headaches when unable to work.
5. Conflict: You feel conflicted with yourself (you know you’re working too much) or with others (partners, friends, and children) about work, but you can’t stop.
6. Relapse: If you manage to cut down your hours but can’t resist overworking 1 day, you wind up right back where you were.
When It’s Time to Address Work Addiction
The lack of a formal diagnosis for work addiction makes getting treatment difficult. But there are ways to seek help. Unlike the drug and alcohol literature, abstinence is not the goal. “The therapeutic goal is getting a behavior under control and looking for the triggers of why you’re compulsively working,” said Dr. Griffiths.
Practice self-compassion
Dr. Sukhera eventually realized that his work addiction stemmed from the fear of being somehow excluded or unworthy. He actively corrected much of this through self-compassion and self-kindness, which helped him set boundaries. “Self-compassion is the root of everything,” he said. “Reminding ourselves that we’re doing our best is an important ingredient in breaking the cycle.”
Slowly expose yourself to relaxation
Many workaholics find rest very difficult. “When I conducted interviews with people [who considered themselves workaholics], a very common thing I heard was, ‘I have a very hard time being idle,’ ” said Dr. Clark. If rest feels hard, Dr. Sukhera suggests practicing relaxation for 2 minutes to start. Even small periods of downtime can challenge the belief that you must be constantly productive.
Reframe your to-do list
For work addicts, to-do lists can seem like they must be finished, which prolongs work hours. Instead, use to-do lists to help prioritize what is urgent, identify what can wait, and delegate out tasks to others, Dr. Clark recommends.
Pick up a mastery experience
Research from professor Sabine Sonnentag, Dr. rer. nat., at the University of Mannheim, Mannheim, Germany, suggests that mastery experiences — leisure activities that require thought and focus like learning a new language or taking a woodworking class — can help you actively disengage from work.
Try cognitive behavioral therapy
Widely used for other forms of addiction, cognitive behavioral therapy centers around recognizing emotions, challenging thought patterns, and changing behaviors. However, Dr. Clark admits the research on its impact on work addiction, in particular, is “pretty nascent.”
Shift your mindset
It seems logical to think that detaching from your feelings will allow you to “do more,” but experts say that idea is both untrue and dangerous. “The safest hospitals are the hospitals where people are attuned to their humanness,” said Dr. Sukhera. “It’s normal to overwork in medicine, and if you’re challenging a norm, you really have to be thoughtful about how you frame that for yourself.”
Most importantly: Seek support
Today, there is increased awareness about work addiction and more resources for physicians who are struggling, including programs such as Workaholics Anonymous or Physicians Anonymous and workplace wellness initiatives. But try not to overwhelm yourself with choosing whom to talk to or what specific resource to utilize, Dr. Sukhera advised. “Just talk to someone about it. You don’t have to carry this on your own.”
A version of this article appeared on Medscape.com.
TEST TEST
test
test
test
Periorbital Changes Induced by Prostaglandin Eye Drops
To the Editor:
A 42-year man presented with hollowing of the upper eyelid and skin discoloration of the left periorbital area of 10 years’ duration. He was a professional mixed martial arts fighter with a history of 2 surgeries for retinal detachment of the left eye 13 years prior to the current presentation. The patient also has macular scarring in the left eye. He denied a history of facial fracture, reconstructive surgery, or other medical conditions. His visual acuity was unknown; however, he did not require corrective glasses. He used 3 prescription ophthalmic eye drops—dorzolamide hydrochloride plus timolol maleate, 10 mL; brimonidine tartrate ophthalmic solution 0.15%, 5 mL; and latanoprost ophthalmic solution 0.005%, 125 μg/2.5 mL—in the left eye to lower intraocular pressure, as therapy for glaucoma. If left untreated, glaucoma can lead to vision loss.
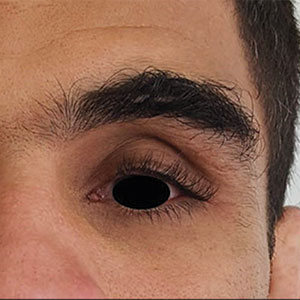
Physical examination revealed periorbital hyperpigmentation on the left side; hypertrichosis and eyelash trichomegaly compared to the right side; and a deep left upper orbital sulcus compared to the right side (Figure). The patient was alert and oriented to person, place, and time. Extraocular movement was intact bilaterally, and his pupillary reflex was symmetric. No tenderness was noted over the affected area on palpation; no subcutaneous masses or lesions were observed or palpated. There was no ocular discharge, the conjunctiva was pink, and the sclera was white bilaterally.
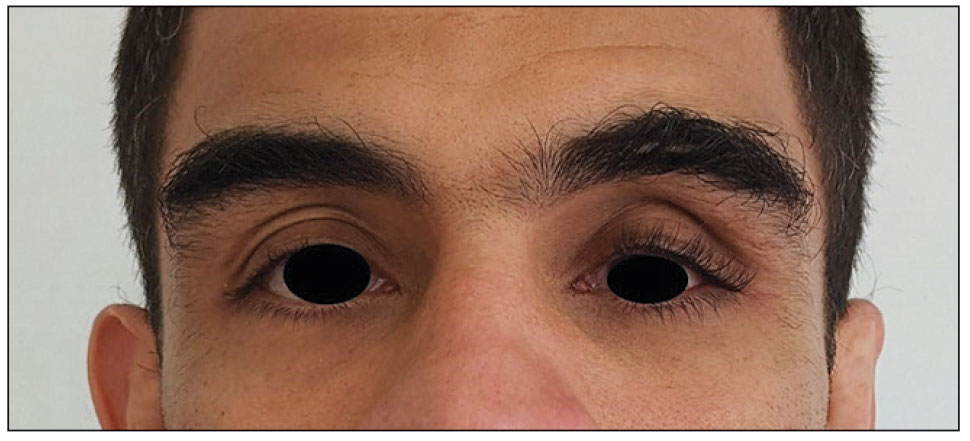
The differential diagnosis included professional trauma-induced orbital changes, nevus of Ota (oculomucodermal melanocytosis), prostaglandin-associated periorbitopathy (PAP), and melasma. Although the patient sustained an injury that caused retinal detachment, he never experienced an orbital bone fracture; additionally, a fracture would not explain the skin discoloration or longer eyelashes. Periorbital nevus of Ota most commonly manifests as a unilateral scleral and brown-bluish skin discoloration but does not cause hollowing of the orbital sulcus or affect the length and thickness of eyelashes. Melasma—bilateral skin hyperpigmentation that most commonly affects women—can be induced by oral contraceptives, antibiotics, heat, sun exposure, and pregnancy. It does not affect the color of the iris or the depth of the scleral sulcus, and it does not increase the length and thickness of eyelashes. Based on the clinical presentation and a review of the eye drops used, he was diagnosed with PAP due to prolonged use of latanoprost ophthalmic solution. The patient was referred to an ophthalmologist for consideration of a switch to a different class of medication.
Of the 3 eye drops used by this patient, latanoprost, a prostaglandin analog, decreases intraocular pressure and is known to cause PAP. This condition comprises a constellation of changes, including upper eyelid ptosis, deepening of the upper eyelid sulcus, involution of dermatochalasis, periorbital fat atrophy, mild enophthalmos (sunken eye), inferior scleral show, increased prominence of eyelid vessels, and tight eyelids.1 Latanoprost most often produces these findings, but all prostaglandin ophthalmic medications can, including the dual-indication bimatoprost, which was approved by the US Food and Drug Administration to reduce elevated intraocular pressure in patients with open-angle glaucoma or ocular hypertension but also is used to grow darker, thicker, and longer eyelashes. Clinicians who prescribe bimatoprost for this cosmetic indication should be mindful of the potential for PAP and discuss it with patients.
The prescribing information (PI) for bimatoprost (Latisse; Allergan) does not list PAP as an adverse reaction observed in the 4-month multicenter, double-blind, randomized, vehicle-controlled study of bimatoprost (as Latisse) in 278 adults.2 The PI does list “periorbital and lid changes associated with periorbital fat atrophy and skin tightness resulting in deepening of eyelid sulcus and eyelid ptosis” as an adverse reaction in postmarketing experience. However, according to the PI, the frequency of these adverse reactions cannot be established, as the reporting of such incidents was voluntary and the size of the treated population was uncertain.2
Prostaglandins can cause periorbitopathy by several mechanisms; one speculated cause is that this group of medications might provoke smooth muscle contraction. Prostaglandin medications also have an affinity for fat cells1; atrophy of fat cells can lead to enophthalmos and deepening upper eyelid sulcus. In an observational study of 105 participants who were using a prostaglandin in 1 eye for longer than 1 month (the other eye was used as a control), the overall frequency of prostaglandin-associated periorbitopathy was 93.3% in the bimatoprost group, 41.4% in the latanoprost group, and 70% in the travoprost group, while the frequency of deepening of the upper eyelid sulcus was 80% in the bimatoprost group, 15.7% in the latanoprost group, and 45% in the travoprost group.3 These changes may not be as striking when a patient is using a prostaglandin ophthalmic medication in both eyes and may not be noticed even by the patient. It is prudent for the clinician to take a baseline photograph of the patient when these medications are prescribed to observe for early signs of periorbitopathy. These adverse effects may not be reversible when the medication is discontinued4 and have been observed as early as 4 to 6 weeks after the start of treatment.5
Our patient was counseled that his constellation of PAP findings potentially could be partially reversed over months or even a year or longer if the offending agent was discontinued. However, he was cautioned that cessation of latanoprost first needed to be discussed with his ophthalmologist, who would determine if there was a suitable alternative to a prostaglandin analog for him. The patient’s only concern was the aesthetic appearance of the left periorbital area. A hyaluronic acid filler or fat grafting can be considered for correction of orbital sulcus hollowing; however, we could not locate any long-term studies in which such corrective treatments were applied for PAP. Our patient continues to use latanoprost with no change in the frequency of use. There have been no further changes or progression in the physical appearance of the left eye or periorbital area. The patient has not undergone any corrective treatments.
- Berke SJ. PAP: new concerns for prostaglandin use. Rev Ophthalmol. 2012;19:70.
- Latisse (bimatoprost ophthalmic solution 0.03%). Package insert. Allergan; 2021. Accessed April 11, 2024. https://www.rxabbvie.com/pdf/latisse_pi.pdf
- Kucukevcilioglu M, Bayer A, Uysal Y, et al. Prostaglandin associated periorbitopathy in patients using bimatoprost, latanoprost and travoprost. Clin Exp Ophthalmol. 2014;42:126-131. doi:10.1111/ceo.12163
- Filippopoulos T, Paula JS, Torun N, et al. Periorbital changes associated with topical bimatoprost. Ophthalmic Plast Reconstr Surg. 2008;24:302-307. doi:10.1097/IOP.0b013e31817d81df
- Peplinski LS, Smith KA. Deepening of lid sulcus from topical bimatoprost therapy. Optom Vis Sci. 2004;81:574-577. doi:10.1097/01.opx.0000141791.16683.4a
To the Editor:
A 42-year man presented with hollowing of the upper eyelid and skin discoloration of the left periorbital area of 10 years’ duration. He was a professional mixed martial arts fighter with a history of 2 surgeries for retinal detachment of the left eye 13 years prior to the current presentation. The patient also has macular scarring in the left eye. He denied a history of facial fracture, reconstructive surgery, or other medical conditions. His visual acuity was unknown; however, he did not require corrective glasses. He used 3 prescription ophthalmic eye drops—dorzolamide hydrochloride plus timolol maleate, 10 mL; brimonidine tartrate ophthalmic solution 0.15%, 5 mL; and latanoprost ophthalmic solution 0.005%, 125 μg/2.5 mL—in the left eye to lower intraocular pressure, as therapy for glaucoma. If left untreated, glaucoma can lead to vision loss.
Physical examination revealed periorbital hyperpigmentation on the left side; hypertrichosis and eyelash trichomegaly compared to the right side; and a deep left upper orbital sulcus compared to the right side (Figure). The patient was alert and oriented to person, place, and time. Extraocular movement was intact bilaterally, and his pupillary reflex was symmetric. No tenderness was noted over the affected area on palpation; no subcutaneous masses or lesions were observed or palpated. There was no ocular discharge, the conjunctiva was pink, and the sclera was white bilaterally.

The differential diagnosis included professional trauma-induced orbital changes, nevus of Ota (oculomucodermal melanocytosis), prostaglandin-associated periorbitopathy (PAP), and melasma. Although the patient sustained an injury that caused retinal detachment, he never experienced an orbital bone fracture; additionally, a fracture would not explain the skin discoloration or longer eyelashes. Periorbital nevus of Ota most commonly manifests as a unilateral scleral and brown-bluish skin discoloration but does not cause hollowing of the orbital sulcus or affect the length and thickness of eyelashes. Melasma—bilateral skin hyperpigmentation that most commonly affects women—can be induced by oral contraceptives, antibiotics, heat, sun exposure, and pregnancy. It does not affect the color of the iris or the depth of the scleral sulcus, and it does not increase the length and thickness of eyelashes. Based on the clinical presentation and a review of the eye drops used, he was diagnosed with PAP due to prolonged use of latanoprost ophthalmic solution. The patient was referred to an ophthalmologist for consideration of a switch to a different class of medication.
Of the 3 eye drops used by this patient, latanoprost, a prostaglandin analog, decreases intraocular pressure and is known to cause PAP. This condition comprises a constellation of changes, including upper eyelid ptosis, deepening of the upper eyelid sulcus, involution of dermatochalasis, periorbital fat atrophy, mild enophthalmos (sunken eye), inferior scleral show, increased prominence of eyelid vessels, and tight eyelids.1 Latanoprost most often produces these findings, but all prostaglandin ophthalmic medications can, including the dual-indication bimatoprost, which was approved by the US Food and Drug Administration to reduce elevated intraocular pressure in patients with open-angle glaucoma or ocular hypertension but also is used to grow darker, thicker, and longer eyelashes. Clinicians who prescribe bimatoprost for this cosmetic indication should be mindful of the potential for PAP and discuss it with patients.
The prescribing information (PI) for bimatoprost (Latisse; Allergan) does not list PAP as an adverse reaction observed in the 4-month multicenter, double-blind, randomized, vehicle-controlled study of bimatoprost (as Latisse) in 278 adults.2 The PI does list “periorbital and lid changes associated with periorbital fat atrophy and skin tightness resulting in deepening of eyelid sulcus and eyelid ptosis” as an adverse reaction in postmarketing experience. However, according to the PI, the frequency of these adverse reactions cannot be established, as the reporting of such incidents was voluntary and the size of the treated population was uncertain.2
Prostaglandins can cause periorbitopathy by several mechanisms; one speculated cause is that this group of medications might provoke smooth muscle contraction. Prostaglandin medications also have an affinity for fat cells1; atrophy of fat cells can lead to enophthalmos and deepening upper eyelid sulcus. In an observational study of 105 participants who were using a prostaglandin in 1 eye for longer than 1 month (the other eye was used as a control), the overall frequency of prostaglandin-associated periorbitopathy was 93.3% in the bimatoprost group, 41.4% in the latanoprost group, and 70% in the travoprost group, while the frequency of deepening of the upper eyelid sulcus was 80% in the bimatoprost group, 15.7% in the latanoprost group, and 45% in the travoprost group.3 These changes may not be as striking when a patient is using a prostaglandin ophthalmic medication in both eyes and may not be noticed even by the patient. It is prudent for the clinician to take a baseline photograph of the patient when these medications are prescribed to observe for early signs of periorbitopathy. These adverse effects may not be reversible when the medication is discontinued4 and have been observed as early as 4 to 6 weeks after the start of treatment.5
Our patient was counseled that his constellation of PAP findings potentially could be partially reversed over months or even a year or longer if the offending agent was discontinued. However, he was cautioned that cessation of latanoprost first needed to be discussed with his ophthalmologist, who would determine if there was a suitable alternative to a prostaglandin analog for him. The patient’s only concern was the aesthetic appearance of the left periorbital area. A hyaluronic acid filler or fat grafting can be considered for correction of orbital sulcus hollowing; however, we could not locate any long-term studies in which such corrective treatments were applied for PAP. Our patient continues to use latanoprost with no change in the frequency of use. There have been no further changes or progression in the physical appearance of the left eye or periorbital area. The patient has not undergone any corrective treatments.
To the Editor:
A 42-year man presented with hollowing of the upper eyelid and skin discoloration of the left periorbital area of 10 years’ duration. He was a professional mixed martial arts fighter with a history of 2 surgeries for retinal detachment of the left eye 13 years prior to the current presentation. The patient also has macular scarring in the left eye. He denied a history of facial fracture, reconstructive surgery, or other medical conditions. His visual acuity was unknown; however, he did not require corrective glasses. He used 3 prescription ophthalmic eye drops—dorzolamide hydrochloride plus timolol maleate, 10 mL; brimonidine tartrate ophthalmic solution 0.15%, 5 mL; and latanoprost ophthalmic solution 0.005%, 125 μg/2.5 mL—in the left eye to lower intraocular pressure, as therapy for glaucoma. If left untreated, glaucoma can lead to vision loss.
Physical examination revealed periorbital hyperpigmentation on the left side; hypertrichosis and eyelash trichomegaly compared to the right side; and a deep left upper orbital sulcus compared to the right side (Figure). The patient was alert and oriented to person, place, and time. Extraocular movement was intact bilaterally, and his pupillary reflex was symmetric. No tenderness was noted over the affected area on palpation; no subcutaneous masses or lesions were observed or palpated. There was no ocular discharge, the conjunctiva was pink, and the sclera was white bilaterally.

The differential diagnosis included professional trauma-induced orbital changes, nevus of Ota (oculomucodermal melanocytosis), prostaglandin-associated periorbitopathy (PAP), and melasma. Although the patient sustained an injury that caused retinal detachment, he never experienced an orbital bone fracture; additionally, a fracture would not explain the skin discoloration or longer eyelashes. Periorbital nevus of Ota most commonly manifests as a unilateral scleral and brown-bluish skin discoloration but does not cause hollowing of the orbital sulcus or affect the length and thickness of eyelashes. Melasma—bilateral skin hyperpigmentation that most commonly affects women—can be induced by oral contraceptives, antibiotics, heat, sun exposure, and pregnancy. It does not affect the color of the iris or the depth of the scleral sulcus, and it does not increase the length and thickness of eyelashes. Based on the clinical presentation and a review of the eye drops used, he was diagnosed with PAP due to prolonged use of latanoprost ophthalmic solution. The patient was referred to an ophthalmologist for consideration of a switch to a different class of medication.
Of the 3 eye drops used by this patient, latanoprost, a prostaglandin analog, decreases intraocular pressure and is known to cause PAP. This condition comprises a constellation of changes, including upper eyelid ptosis, deepening of the upper eyelid sulcus, involution of dermatochalasis, periorbital fat atrophy, mild enophthalmos (sunken eye), inferior scleral show, increased prominence of eyelid vessels, and tight eyelids.1 Latanoprost most often produces these findings, but all prostaglandin ophthalmic medications can, including the dual-indication bimatoprost, which was approved by the US Food and Drug Administration to reduce elevated intraocular pressure in patients with open-angle glaucoma or ocular hypertension but also is used to grow darker, thicker, and longer eyelashes. Clinicians who prescribe bimatoprost for this cosmetic indication should be mindful of the potential for PAP and discuss it with patients.
The prescribing information (PI) for bimatoprost (Latisse; Allergan) does not list PAP as an adverse reaction observed in the 4-month multicenter, double-blind, randomized, vehicle-controlled study of bimatoprost (as Latisse) in 278 adults.2 The PI does list “periorbital and lid changes associated with periorbital fat atrophy and skin tightness resulting in deepening of eyelid sulcus and eyelid ptosis” as an adverse reaction in postmarketing experience. However, according to the PI, the frequency of these adverse reactions cannot be established, as the reporting of such incidents was voluntary and the size of the treated population was uncertain.2
Prostaglandins can cause periorbitopathy by several mechanisms; one speculated cause is that this group of medications might provoke smooth muscle contraction. Prostaglandin medications also have an affinity for fat cells1; atrophy of fat cells can lead to enophthalmos and deepening upper eyelid sulcus. In an observational study of 105 participants who were using a prostaglandin in 1 eye for longer than 1 month (the other eye was used as a control), the overall frequency of prostaglandin-associated periorbitopathy was 93.3% in the bimatoprost group, 41.4% in the latanoprost group, and 70% in the travoprost group, while the frequency of deepening of the upper eyelid sulcus was 80% in the bimatoprost group, 15.7% in the latanoprost group, and 45% in the travoprost group.3 These changes may not be as striking when a patient is using a prostaglandin ophthalmic medication in both eyes and may not be noticed even by the patient. It is prudent for the clinician to take a baseline photograph of the patient when these medications are prescribed to observe for early signs of periorbitopathy. These adverse effects may not be reversible when the medication is discontinued4 and have been observed as early as 4 to 6 weeks after the start of treatment.5
Our patient was counseled that his constellation of PAP findings potentially could be partially reversed over months or even a year or longer if the offending agent was discontinued. However, he was cautioned that cessation of latanoprost first needed to be discussed with his ophthalmologist, who would determine if there was a suitable alternative to a prostaglandin analog for him. The patient’s only concern was the aesthetic appearance of the left periorbital area. A hyaluronic acid filler or fat grafting can be considered for correction of orbital sulcus hollowing; however, we could not locate any long-term studies in which such corrective treatments were applied for PAP. Our patient continues to use latanoprost with no change in the frequency of use. There have been no further changes or progression in the physical appearance of the left eye or periorbital area. The patient has not undergone any corrective treatments.
- Berke SJ. PAP: new concerns for prostaglandin use. Rev Ophthalmol. 2012;19:70.
- Latisse (bimatoprost ophthalmic solution 0.03%). Package insert. Allergan; 2021. Accessed April 11, 2024. https://www.rxabbvie.com/pdf/latisse_pi.pdf
- Kucukevcilioglu M, Bayer A, Uysal Y, et al. Prostaglandin associated periorbitopathy in patients using bimatoprost, latanoprost and travoprost. Clin Exp Ophthalmol. 2014;42:126-131. doi:10.1111/ceo.12163
- Filippopoulos T, Paula JS, Torun N, et al. Periorbital changes associated with topical bimatoprost. Ophthalmic Plast Reconstr Surg. 2008;24:302-307. doi:10.1097/IOP.0b013e31817d81df
- Peplinski LS, Smith KA. Deepening of lid sulcus from topical bimatoprost therapy. Optom Vis Sci. 2004;81:574-577. doi:10.1097/01.opx.0000141791.16683.4a
- Berke SJ. PAP: new concerns for prostaglandin use. Rev Ophthalmol. 2012;19:70.
- Latisse (bimatoprost ophthalmic solution 0.03%). Package insert. Allergan; 2021. Accessed April 11, 2024. https://www.rxabbvie.com/pdf/latisse_pi.pdf
- Kucukevcilioglu M, Bayer A, Uysal Y, et al. Prostaglandin associated periorbitopathy in patients using bimatoprost, latanoprost and travoprost. Clin Exp Ophthalmol. 2014;42:126-131. doi:10.1111/ceo.12163
- Filippopoulos T, Paula JS, Torun N, et al. Periorbital changes associated with topical bimatoprost. Ophthalmic Plast Reconstr Surg. 2008;24:302-307. doi:10.1097/IOP.0b013e31817d81df
- Peplinski LS, Smith KA. Deepening of lid sulcus from topical bimatoprost therapy. Optom Vis Sci. 2004;81:574-577. doi:10.1097/01.opx.0000141791.16683.4a
PRACTICE POINTS
- Ask patients to provide photographs taken prior to noticed changes to assess progression if they are new to your practice.
- Take photographs of patients in good light against a solid-colored background to have a baseline. It may be helpful to update patient images annually.
- Discuss with patients the aesthetic changes that may occur with the use of prescription medications. Provide pamphlets with images to educate them on what to expect.
Late-Stage Incidence Rates Support CRC Screening From Age 45
, a cross-sectional study of stage-stratified CRC found.
It is well known that CRC is becoming more prevalent generally in the under 50-year population, but stage-related analyses have not been done.
Staging analysis in this age group is important, however, as an increasing burden of advance-staged disease would provide further evidence for earlier screening initiation, wrote Eric M. Montminy, MD, a gastroenterologist at John H. Stroger Hospital of County Cook, Chicago, Illinois, and colleagues in JAMA Network Open.
The United States Preventive Services Task Force (USPSTF) has recommended that average-risk screening begin at 45 years of age, as do the American Gastroenterological Association and other GI societies, although the American College of Physicians last year published clinical guidance recommending 50 years as the age to start screening for CRC for patients with average risk.
“Patients aged 46-49 may become confused on which guideline to follow, similar to confusion occurring with prior breast cancer screening changes,” Dr. Montminy said in an interview. “We wanted to demonstrate incidence rates with stage stratification to help clarify the incidence trends in this age group. Stage stratification is a key because it provides insight into the relationship between time and cancer incidence, ie, is screening finding early cancer or not?”
A 2020 study in JAMA Network Open demonstrated a 46.1% increase in CRC incidence rates (IRs) in persons aged 49-50 years. This steep increase is consistent with the presence of a large preexisting and undetected case burden.
“Our results demonstrate that adults aged 46-49 years, who are between now-conflicting guidelines on whether to start screening at age 45 or 50 years, have an increasing burden of more advanced-stage CRC and thus may be at an increased risk if screening is not initiated at age 45 years,” Dr. Montminy’s group wrote.
Using incidence data per 100,000 population from the National Cancer Institute’s Surveillance, Epidemiology, and End Results registry, the investigators observed the following IRs for early-onset CRC in the age group of 46-49 years:
- Distant adenocarcinoma IRs increased faster than other stages: annual percentage change (APC), 2.2 (95% CI, 1.8-2.6).
- Regional IRs also significantly increased: APC, 1.3 (95% CI, 0.8-1.7).
- Absolute regional IRs of CRC in the age bracket of 46-49 years are similar to total pancreatic cancer IRs in all ages and all stages combined (13.2 of 100,000) over similar years. When distant IRs for CRC are included with regional IRs, those for IRs for CRC are double those for pancreatic cancer of all stages combined.
- The only decrease was seen in localized IRs: APC, -0.6 (95% CI, -1 to -0.2).
“My best advice for clinicians is to provide the facts from the data to patients so they can make an informed health decision,” Dr. Montminy said. “This includes taking an appropriate personal and family history and having the patient factor this aspect into their decision on when and how they want to perform colon cancer screening.”
His institution adheres to the USPSTF recommendation of initiation of CRC screening at age 45 years.
Findings From 2000 to 2020
During 2000-2020 period, 26,887 CRCs were diagnosed in adults aged 46-49 years (54.5% in men).
As of 2020, the localized adenocarcinoma IR decreased to 7.7 of 100,000, but regional adenocarcinoma IR increased to 13.4 of 100,000 and distant adenocarcinoma IR increased to 9.0 of 100,000.
Regional adenocarcinoma IR remained the highest of all stages in 2000-2020. From 2014 to 2020, distant IRs became similar to localized IRs, except in 2017 when distant IRs were significantly higher than localized.
Why the CRC Uptick?
“It remains an enigma at this time as to why we’re seeing this shift,” Dr. Montminy said, noting that etiologies from the colonic microbiome to cellphones have been postulated. “To date, no theory has substantially provided causality. But whatever the source is, it is affecting Western countries in unison with data demonstrating a birth cohort effect as well,” he added. “We additionally know, based on the current epidemiologic data, that current screening practices are failing, and a unified discussion must occur in order to prevent young patients from developing advanced colon cancer.”
Offering his perspective on the findings, Joshua Meyer, MD, vice chair of translational research in the Department of Radiation Oncology at Fox Chase Cancer Center in Philadelphia, said the findings reinforce the practice of offering screening to average-risk individuals starting at age 45 years, the threshold at his institution. “There are previously published data demonstrating an increase in advanced stage at the time of screening initiation, and these data support that,” said Dr. Meyer, who was not involved in the present analysis.
More research needs to be done, he continued, not just on optimal age but also on the effect of multiple other factors impacting risk. “These may include family history and genetic risk as well as the role of blood- and stool-based screening assays in an integrated strategy to screen for colorectal cancer.”
There are multiple screening tests, and while colonoscopy, the gold standard, is very safe, it is not completely without risks, Dr. Meyer added. “And the question of the appropriate allocation of limited societal resources continues to be discussed on a broader level and largely explains the difference between the two guidelines.”
This study received no specific funding. Co-author Jordan J. Karlitz, MD, reported personal fees from GRAIL (senior medical director) and an equity position from Gastro Girl/GI On Demand outside f the submitted work. Dr. Meyer disclosed no conflicts of interest relevant to his comments.
, a cross-sectional study of stage-stratified CRC found.
It is well known that CRC is becoming more prevalent generally in the under 50-year population, but stage-related analyses have not been done.
Staging analysis in this age group is important, however, as an increasing burden of advance-staged disease would provide further evidence for earlier screening initiation, wrote Eric M. Montminy, MD, a gastroenterologist at John H. Stroger Hospital of County Cook, Chicago, Illinois, and colleagues in JAMA Network Open.
The United States Preventive Services Task Force (USPSTF) has recommended that average-risk screening begin at 45 years of age, as do the American Gastroenterological Association and other GI societies, although the American College of Physicians last year published clinical guidance recommending 50 years as the age to start screening for CRC for patients with average risk.
“Patients aged 46-49 may become confused on which guideline to follow, similar to confusion occurring with prior breast cancer screening changes,” Dr. Montminy said in an interview. “We wanted to demonstrate incidence rates with stage stratification to help clarify the incidence trends in this age group. Stage stratification is a key because it provides insight into the relationship between time and cancer incidence, ie, is screening finding early cancer or not?”
A 2020 study in JAMA Network Open demonstrated a 46.1% increase in CRC incidence rates (IRs) in persons aged 49-50 years. This steep increase is consistent with the presence of a large preexisting and undetected case burden.
“Our results demonstrate that adults aged 46-49 years, who are between now-conflicting guidelines on whether to start screening at age 45 or 50 years, have an increasing burden of more advanced-stage CRC and thus may be at an increased risk if screening is not initiated at age 45 years,” Dr. Montminy’s group wrote.
Using incidence data per 100,000 population from the National Cancer Institute’s Surveillance, Epidemiology, and End Results registry, the investigators observed the following IRs for early-onset CRC in the age group of 46-49 years:
- Distant adenocarcinoma IRs increased faster than other stages: annual percentage change (APC), 2.2 (95% CI, 1.8-2.6).
- Regional IRs also significantly increased: APC, 1.3 (95% CI, 0.8-1.7).
- Absolute regional IRs of CRC in the age bracket of 46-49 years are similar to total pancreatic cancer IRs in all ages and all stages combined (13.2 of 100,000) over similar years. When distant IRs for CRC are included with regional IRs, those for IRs for CRC are double those for pancreatic cancer of all stages combined.
- The only decrease was seen in localized IRs: APC, -0.6 (95% CI, -1 to -0.2).
“My best advice for clinicians is to provide the facts from the data to patients so they can make an informed health decision,” Dr. Montminy said. “This includes taking an appropriate personal and family history and having the patient factor this aspect into their decision on when and how they want to perform colon cancer screening.”
His institution adheres to the USPSTF recommendation of initiation of CRC screening at age 45 years.
Findings From 2000 to 2020
During 2000-2020 period, 26,887 CRCs were diagnosed in adults aged 46-49 years (54.5% in men).
As of 2020, the localized adenocarcinoma IR decreased to 7.7 of 100,000, but regional adenocarcinoma IR increased to 13.4 of 100,000 and distant adenocarcinoma IR increased to 9.0 of 100,000.
Regional adenocarcinoma IR remained the highest of all stages in 2000-2020. From 2014 to 2020, distant IRs became similar to localized IRs, except in 2017 when distant IRs were significantly higher than localized.
Why the CRC Uptick?
“It remains an enigma at this time as to why we’re seeing this shift,” Dr. Montminy said, noting that etiologies from the colonic microbiome to cellphones have been postulated. “To date, no theory has substantially provided causality. But whatever the source is, it is affecting Western countries in unison with data demonstrating a birth cohort effect as well,” he added. “We additionally know, based on the current epidemiologic data, that current screening practices are failing, and a unified discussion must occur in order to prevent young patients from developing advanced colon cancer.”
Offering his perspective on the findings, Joshua Meyer, MD, vice chair of translational research in the Department of Radiation Oncology at Fox Chase Cancer Center in Philadelphia, said the findings reinforce the practice of offering screening to average-risk individuals starting at age 45 years, the threshold at his institution. “There are previously published data demonstrating an increase in advanced stage at the time of screening initiation, and these data support that,” said Dr. Meyer, who was not involved in the present analysis.
More research needs to be done, he continued, not just on optimal age but also on the effect of multiple other factors impacting risk. “These may include family history and genetic risk as well as the role of blood- and stool-based screening assays in an integrated strategy to screen for colorectal cancer.”
There are multiple screening tests, and while colonoscopy, the gold standard, is very safe, it is not completely without risks, Dr. Meyer added. “And the question of the appropriate allocation of limited societal resources continues to be discussed on a broader level and largely explains the difference between the two guidelines.”
This study received no specific funding. Co-author Jordan J. Karlitz, MD, reported personal fees from GRAIL (senior medical director) and an equity position from Gastro Girl/GI On Demand outside f the submitted work. Dr. Meyer disclosed no conflicts of interest relevant to his comments.
, a cross-sectional study of stage-stratified CRC found.
It is well known that CRC is becoming more prevalent generally in the under 50-year population, but stage-related analyses have not been done.
Staging analysis in this age group is important, however, as an increasing burden of advance-staged disease would provide further evidence for earlier screening initiation, wrote Eric M. Montminy, MD, a gastroenterologist at John H. Stroger Hospital of County Cook, Chicago, Illinois, and colleagues in JAMA Network Open.
The United States Preventive Services Task Force (USPSTF) has recommended that average-risk screening begin at 45 years of age, as do the American Gastroenterological Association and other GI societies, although the American College of Physicians last year published clinical guidance recommending 50 years as the age to start screening for CRC for patients with average risk.
“Patients aged 46-49 may become confused on which guideline to follow, similar to confusion occurring with prior breast cancer screening changes,” Dr. Montminy said in an interview. “We wanted to demonstrate incidence rates with stage stratification to help clarify the incidence trends in this age group. Stage stratification is a key because it provides insight into the relationship between time and cancer incidence, ie, is screening finding early cancer or not?”
A 2020 study in JAMA Network Open demonstrated a 46.1% increase in CRC incidence rates (IRs) in persons aged 49-50 years. This steep increase is consistent with the presence of a large preexisting and undetected case burden.
“Our results demonstrate that adults aged 46-49 years, who are between now-conflicting guidelines on whether to start screening at age 45 or 50 years, have an increasing burden of more advanced-stage CRC and thus may be at an increased risk if screening is not initiated at age 45 years,” Dr. Montminy’s group wrote.
Using incidence data per 100,000 population from the National Cancer Institute’s Surveillance, Epidemiology, and End Results registry, the investigators observed the following IRs for early-onset CRC in the age group of 46-49 years:
- Distant adenocarcinoma IRs increased faster than other stages: annual percentage change (APC), 2.2 (95% CI, 1.8-2.6).
- Regional IRs also significantly increased: APC, 1.3 (95% CI, 0.8-1.7).
- Absolute regional IRs of CRC in the age bracket of 46-49 years are similar to total pancreatic cancer IRs in all ages and all stages combined (13.2 of 100,000) over similar years. When distant IRs for CRC are included with regional IRs, those for IRs for CRC are double those for pancreatic cancer of all stages combined.
- The only decrease was seen in localized IRs: APC, -0.6 (95% CI, -1 to -0.2).
“My best advice for clinicians is to provide the facts from the data to patients so they can make an informed health decision,” Dr. Montminy said. “This includes taking an appropriate personal and family history and having the patient factor this aspect into their decision on when and how they want to perform colon cancer screening.”
His institution adheres to the USPSTF recommendation of initiation of CRC screening at age 45 years.
Findings From 2000 to 2020
During 2000-2020 period, 26,887 CRCs were diagnosed in adults aged 46-49 years (54.5% in men).
As of 2020, the localized adenocarcinoma IR decreased to 7.7 of 100,000, but regional adenocarcinoma IR increased to 13.4 of 100,000 and distant adenocarcinoma IR increased to 9.0 of 100,000.
Regional adenocarcinoma IR remained the highest of all stages in 2000-2020. From 2014 to 2020, distant IRs became similar to localized IRs, except in 2017 when distant IRs were significantly higher than localized.
Why the CRC Uptick?
“It remains an enigma at this time as to why we’re seeing this shift,” Dr. Montminy said, noting that etiologies from the colonic microbiome to cellphones have been postulated. “To date, no theory has substantially provided causality. But whatever the source is, it is affecting Western countries in unison with data demonstrating a birth cohort effect as well,” he added. “We additionally know, based on the current epidemiologic data, that current screening practices are failing, and a unified discussion must occur in order to prevent young patients from developing advanced colon cancer.”
Offering his perspective on the findings, Joshua Meyer, MD, vice chair of translational research in the Department of Radiation Oncology at Fox Chase Cancer Center in Philadelphia, said the findings reinforce the practice of offering screening to average-risk individuals starting at age 45 years, the threshold at his institution. “There are previously published data demonstrating an increase in advanced stage at the time of screening initiation, and these data support that,” said Dr. Meyer, who was not involved in the present analysis.
More research needs to be done, he continued, not just on optimal age but also on the effect of multiple other factors impacting risk. “These may include family history and genetic risk as well as the role of blood- and stool-based screening assays in an integrated strategy to screen for colorectal cancer.”
There are multiple screening tests, and while colonoscopy, the gold standard, is very safe, it is not completely without risks, Dr. Meyer added. “And the question of the appropriate allocation of limited societal resources continues to be discussed on a broader level and largely explains the difference between the two guidelines.”
This study received no specific funding. Co-author Jordan J. Karlitz, MD, reported personal fees from GRAIL (senior medical director) and an equity position from Gastro Girl/GI On Demand outside f the submitted work. Dr. Meyer disclosed no conflicts of interest relevant to his comments.
FROM JAMA NETWORK OPEN
Commentary: Diet and Lifestyle in Migraine, May 2024
Migraine and other headache types are common ailments, and there are many stereotypes and stigmas associated with these conditions. One of the prevailing beliefs about headaches and migraines is that they are linked with internalizing mental health conditions — anxiety and depression. These associations can affect pediatric migraine patients and their parents in complicated ways, potentially hindering adequate diagnosis and treatment. Results of a recent prospective study, published in the journal Headache, provided results that challenge the widespread belief that people who have migraines have a higher-than-average rate of internalizing mental health disorders. The authors provided a discussion and data to explain that their initial hypothesis of a relationship between migraine and mental health was disproven. The study included 123 participants age 8-18 years who had been previously diagnosed with migraine. The patients, who were seen in a pediatric neurology clinic, completed headache questionnaires and validated measures of anxiety and depressive symptoms. The final analysis showed no significant association between migraines or headaches with anxiety or depression.
Why does this matter? Stigma can prevent patients and parents from seeking care if parents feel that they will be judged as bad parents for contributing to their children's anxiety, depression, headaches, and migraines. In fact, beyond mental health stigma, children who have migraine can be blamed for having an unhealthy lifestyle.[1] While advice to get enough sleep, eat healthy, and stay active is worthwhile, there can be an implication that pediatric migraine patients are causing their migraines by living an unhealthy lifestyle.[1] Additionally, the implication that parents are not properly taking care of their children's health can inhibit an accurate symptom history. Releasing pediatric migraine patients and their parents from myths about migraines and headaches can be a beneficial component of doctor-patient communication regarding migraine care.
It is possible that dietary adjustments or supplements could help improve migraine frequency and severity. Maintaining a healthy diet is a frequent recommendation for people who have headaches, but it can be frustrating for patients to receive general recommendations to follow a healthy lifestyle. Specific direction regarding which foods to avoid and which foods to add to a diet can be helpful for patients as they try to navigate the challenge of adopting migraine-friendly lifestyle changes.
Eicosapentaenoic acid (EPA) is one of the omega-3 fatty acids. A recent study, with results published in Brain, Behavior, and Immunity, examined the effects of EPA on migraines. The 12-week randomized, double-blind, placebo-controlled trial included 70 participants who had been diagnosed with episodic migraine. Participants were randomly assigned to either EPA (2 g fish oil with 1.8 g of EPA/day) or placebo (2 g soybean oil/day). Migraine frequency and severity were assessed using standardized scales. According to the authors, the high-dose-EPA group had significantly reduced migraine frequency and severity, fewer number of days using acute treatment, reduced migraine-associated disability, improved anxiety and depression, and improved quality of life in comparison to the placebo group. The EPA group did not experience notable adverse events.To provide a sense of scale regarding dietary EPA, 3 oz of cooked wild salmon has 0.35 g of EPA, 3 oz of cooked shrimp has 0.2 g of EPA, and 3 oz of light canned tuna has 0.02 g of EPA.[2] Thus, it's important to note that the amount of EPA used in this study was higher than what would be expected of dietary EPA.
An observational prospective study published in Scientific Reports examined the effects of dietary phytochemical index (DPI) on migraine. DPI is defined as the proportion of daily energy intake derived from foods rich in phytochemicals. Consumption of phytochemical-rich foods has been associated with cardiovascular and metabolic diseases prevention in various populations. These foods include fruits, vegetables, whole grains, seeds, nuts, and legumes. The study included 265 adults age 20-50 who had a diagnosis of migraine. Participants were asked to fill out a questionnaire, which was used to evaluate their diet in the preceding year, and they were asked to complete a diary to track their migraine symptoms. The results showed an inverse relationship between DPI index and migraine frequency. Participants who had the highest DPI had the lowest migraine frequency.[3] While the authors found the results to be statistically significant, they did not point to a cause and effect. Migraine-associated symptoms such as nausea can have an effect on dietary choices, so patients who experience migraine symptoms may avoid certain foods before, during, or after a migraine episode. They also may consistently avoid foods that they have experienced as migraine triggers.
Diet and lifestyle can have an effect on migraine frequency, severity, and overall migraine-associated quality of life. Beyond general recommendations, however, it is not yet well established which foods or supplements could potentially help alleviate migraines. Advice to maintain a healthy lifestyle is definitely worthwhile for migraine patients, but it is important to avoid conveying blame or stigma when it comes to communication about the effect of lifestyle on migraine. This is especially important for pediatric migraine patients because the stigma extends beyond children to parents and could potentially interfere with clear communication and adequate care.
Additional References
1. Gelfand AA, Irwin SL. Lifestyle advice for pediatric migraine: Blaming the patient, or evidence based? Semin Neurol. 2020;40:277-285. doi: 10.1055/s-0040-1708868 Source
2. National Institutes of Health. Office of Dietary Supplements. Omega-3 fatty acids. Updated February 15, 2023. Source
3. Hamedi-Shahraki S, Jowshan M-R, Zolghadrpour M-A, et al. Dietary phytochemical index is favorably associated with oxidative stress status and cardiovascular risk factors in adults with obesity. Sci Rep. 2023;13:7035. doi: 10.1038/s41598-023-34064-4 Source
Migraine and other headache types are common ailments, and there are many stereotypes and stigmas associated with these conditions. One of the prevailing beliefs about headaches and migraines is that they are linked with internalizing mental health conditions — anxiety and depression. These associations can affect pediatric migraine patients and their parents in complicated ways, potentially hindering adequate diagnosis and treatment. Results of a recent prospective study, published in the journal Headache, provided results that challenge the widespread belief that people who have migraines have a higher-than-average rate of internalizing mental health disorders. The authors provided a discussion and data to explain that their initial hypothesis of a relationship between migraine and mental health was disproven. The study included 123 participants age 8-18 years who had been previously diagnosed with migraine. The patients, who were seen in a pediatric neurology clinic, completed headache questionnaires and validated measures of anxiety and depressive symptoms. The final analysis showed no significant association between migraines or headaches with anxiety or depression.
Why does this matter? Stigma can prevent patients and parents from seeking care if parents feel that they will be judged as bad parents for contributing to their children's anxiety, depression, headaches, and migraines. In fact, beyond mental health stigma, children who have migraine can be blamed for having an unhealthy lifestyle.[1] While advice to get enough sleep, eat healthy, and stay active is worthwhile, there can be an implication that pediatric migraine patients are causing their migraines by living an unhealthy lifestyle.[1] Additionally, the implication that parents are not properly taking care of their children's health can inhibit an accurate symptom history. Releasing pediatric migraine patients and their parents from myths about migraines and headaches can be a beneficial component of doctor-patient communication regarding migraine care.
It is possible that dietary adjustments or supplements could help improve migraine frequency and severity. Maintaining a healthy diet is a frequent recommendation for people who have headaches, but it can be frustrating for patients to receive general recommendations to follow a healthy lifestyle. Specific direction regarding which foods to avoid and which foods to add to a diet can be helpful for patients as they try to navigate the challenge of adopting migraine-friendly lifestyle changes.
Eicosapentaenoic acid (EPA) is one of the omega-3 fatty acids. A recent study, with results published in Brain, Behavior, and Immunity, examined the effects of EPA on migraines. The 12-week randomized, double-blind, placebo-controlled trial included 70 participants who had been diagnosed with episodic migraine. Participants were randomly assigned to either EPA (2 g fish oil with 1.8 g of EPA/day) or placebo (2 g soybean oil/day). Migraine frequency and severity were assessed using standardized scales. According to the authors, the high-dose-EPA group had significantly reduced migraine frequency and severity, fewer number of days using acute treatment, reduced migraine-associated disability, improved anxiety and depression, and improved quality of life in comparison to the placebo group. The EPA group did not experience notable adverse events.To provide a sense of scale regarding dietary EPA, 3 oz of cooked wild salmon has 0.35 g of EPA, 3 oz of cooked shrimp has 0.2 g of EPA, and 3 oz of light canned tuna has 0.02 g of EPA.[2] Thus, it's important to note that the amount of EPA used in this study was higher than what would be expected of dietary EPA.
An observational prospective study published in Scientific Reports examined the effects of dietary phytochemical index (DPI) on migraine. DPI is defined as the proportion of daily energy intake derived from foods rich in phytochemicals. Consumption of phytochemical-rich foods has been associated with cardiovascular and metabolic diseases prevention in various populations. These foods include fruits, vegetables, whole grains, seeds, nuts, and legumes. The study included 265 adults age 20-50 who had a diagnosis of migraine. Participants were asked to fill out a questionnaire, which was used to evaluate their diet in the preceding year, and they were asked to complete a diary to track their migraine symptoms. The results showed an inverse relationship between DPI index and migraine frequency. Participants who had the highest DPI had the lowest migraine frequency.[3] While the authors found the results to be statistically significant, they did not point to a cause and effect. Migraine-associated symptoms such as nausea can have an effect on dietary choices, so patients who experience migraine symptoms may avoid certain foods before, during, or after a migraine episode. They also may consistently avoid foods that they have experienced as migraine triggers.
Diet and lifestyle can have an effect on migraine frequency, severity, and overall migraine-associated quality of life. Beyond general recommendations, however, it is not yet well established which foods or supplements could potentially help alleviate migraines. Advice to maintain a healthy lifestyle is definitely worthwhile for migraine patients, but it is important to avoid conveying blame or stigma when it comes to communication about the effect of lifestyle on migraine. This is especially important for pediatric migraine patients because the stigma extends beyond children to parents and could potentially interfere with clear communication and adequate care.
Additional References
1. Gelfand AA, Irwin SL. Lifestyle advice for pediatric migraine: Blaming the patient, or evidence based? Semin Neurol. 2020;40:277-285. doi: 10.1055/s-0040-1708868 Source
2. National Institutes of Health. Office of Dietary Supplements. Omega-3 fatty acids. Updated February 15, 2023. Source
3. Hamedi-Shahraki S, Jowshan M-R, Zolghadrpour M-A, et al. Dietary phytochemical index is favorably associated with oxidative stress status and cardiovascular risk factors in adults with obesity. Sci Rep. 2023;13:7035. doi: 10.1038/s41598-023-34064-4 Source
Migraine and other headache types are common ailments, and there are many stereotypes and stigmas associated with these conditions. One of the prevailing beliefs about headaches and migraines is that they are linked with internalizing mental health conditions — anxiety and depression. These associations can affect pediatric migraine patients and their parents in complicated ways, potentially hindering adequate diagnosis and treatment. Results of a recent prospective study, published in the journal Headache, provided results that challenge the widespread belief that people who have migraines have a higher-than-average rate of internalizing mental health disorders. The authors provided a discussion and data to explain that their initial hypothesis of a relationship between migraine and mental health was disproven. The study included 123 participants age 8-18 years who had been previously diagnosed with migraine. The patients, who were seen in a pediatric neurology clinic, completed headache questionnaires and validated measures of anxiety and depressive symptoms. The final analysis showed no significant association between migraines or headaches with anxiety or depression.
Why does this matter? Stigma can prevent patients and parents from seeking care if parents feel that they will be judged as bad parents for contributing to their children's anxiety, depression, headaches, and migraines. In fact, beyond mental health stigma, children who have migraine can be blamed for having an unhealthy lifestyle.[1] While advice to get enough sleep, eat healthy, and stay active is worthwhile, there can be an implication that pediatric migraine patients are causing their migraines by living an unhealthy lifestyle.[1] Additionally, the implication that parents are not properly taking care of their children's health can inhibit an accurate symptom history. Releasing pediatric migraine patients and their parents from myths about migraines and headaches can be a beneficial component of doctor-patient communication regarding migraine care.
It is possible that dietary adjustments or supplements could help improve migraine frequency and severity. Maintaining a healthy diet is a frequent recommendation for people who have headaches, but it can be frustrating for patients to receive general recommendations to follow a healthy lifestyle. Specific direction regarding which foods to avoid and which foods to add to a diet can be helpful for patients as they try to navigate the challenge of adopting migraine-friendly lifestyle changes.
Eicosapentaenoic acid (EPA) is one of the omega-3 fatty acids. A recent study, with results published in Brain, Behavior, and Immunity, examined the effects of EPA on migraines. The 12-week randomized, double-blind, placebo-controlled trial included 70 participants who had been diagnosed with episodic migraine. Participants were randomly assigned to either EPA (2 g fish oil with 1.8 g of EPA/day) or placebo (2 g soybean oil/day). Migraine frequency and severity were assessed using standardized scales. According to the authors, the high-dose-EPA group had significantly reduced migraine frequency and severity, fewer number of days using acute treatment, reduced migraine-associated disability, improved anxiety and depression, and improved quality of life in comparison to the placebo group. The EPA group did not experience notable adverse events.To provide a sense of scale regarding dietary EPA, 3 oz of cooked wild salmon has 0.35 g of EPA, 3 oz of cooked shrimp has 0.2 g of EPA, and 3 oz of light canned tuna has 0.02 g of EPA.[2] Thus, it's important to note that the amount of EPA used in this study was higher than what would be expected of dietary EPA.
An observational prospective study published in Scientific Reports examined the effects of dietary phytochemical index (DPI) on migraine. DPI is defined as the proportion of daily energy intake derived from foods rich in phytochemicals. Consumption of phytochemical-rich foods has been associated with cardiovascular and metabolic diseases prevention in various populations. These foods include fruits, vegetables, whole grains, seeds, nuts, and legumes. The study included 265 adults age 20-50 who had a diagnosis of migraine. Participants were asked to fill out a questionnaire, which was used to evaluate their diet in the preceding year, and they were asked to complete a diary to track their migraine symptoms. The results showed an inverse relationship between DPI index and migraine frequency. Participants who had the highest DPI had the lowest migraine frequency.[3] While the authors found the results to be statistically significant, they did not point to a cause and effect. Migraine-associated symptoms such as nausea can have an effect on dietary choices, so patients who experience migraine symptoms may avoid certain foods before, during, or after a migraine episode. They also may consistently avoid foods that they have experienced as migraine triggers.
Diet and lifestyle can have an effect on migraine frequency, severity, and overall migraine-associated quality of life. Beyond general recommendations, however, it is not yet well established which foods or supplements could potentially help alleviate migraines. Advice to maintain a healthy lifestyle is definitely worthwhile for migraine patients, but it is important to avoid conveying blame or stigma when it comes to communication about the effect of lifestyle on migraine. This is especially important for pediatric migraine patients because the stigma extends beyond children to parents and could potentially interfere with clear communication and adequate care.
Additional References
1. Gelfand AA, Irwin SL. Lifestyle advice for pediatric migraine: Blaming the patient, or evidence based? Semin Neurol. 2020;40:277-285. doi: 10.1055/s-0040-1708868 Source
2. National Institutes of Health. Office of Dietary Supplements. Omega-3 fatty acids. Updated February 15, 2023. Source
3. Hamedi-Shahraki S, Jowshan M-R, Zolghadrpour M-A, et al. Dietary phytochemical index is favorably associated with oxidative stress status and cardiovascular risk factors in adults with obesity. Sci Rep. 2023;13:7035. doi: 10.1038/s41598-023-34064-4 Source
Could Bedside Training Help End the US Neurologist Shortage?
DENVER — , a new report suggested.
Bedside Rounding Alliance for Internal Medicine and Neurology Residents (BRAINs) moves training from the lecture hall to the bedside, offering instruction on obtaining a focused neurologic history and performing a focused neurologic physical exam for common neurologic symptoms.
Almost 100% of trainees surveyed gave the program a favorable rating, citing patient exposure and bedside training from neurology educators as keys to its success.
As internal medicine providers are often “the first to lay eyes” on patients with a neurology complaint, it’s important they “have a basic level of comfort” in addressing patients’ common questions and concerns, study author Prashanth Rajarajan, MD, PhD, a resident in the Department of Neurology at Brigham and Women’s Hospital, Boston, told this news organization.
The findings were presented at the 2024 annual meeting of the American Academy of Neurology.
Addressing ‘Neurophobia’
Neurology is often viewed by medical trainees as the most difficult subspecialty, Dr. Rajarajan said. Many have what he calls “neurophobia,” which he defines as “a discomfort with assessing and treating neurologic complaints.”
A survey at his institution showed 62% of internal medicine residents lacked the confidence to diagnose and treat neurologic diseases, he reported.
BRAINs is a structured neurology trainee-led, inpatient bedside teaching session for internal medicine residents, medical students, and others that aims to increase trainees’ confidence in assessing patients with common neurologic symptoms.
The program includes a biweekly 45-minute session. Most of the session is spent at the bedside and involves demonstrations and practice of a focused neurologic history and physical exam.
Participants receive feedback from educators, typically neurology residents or fellows in epilepsy, stroke, or some other neurology subspecialty. It also includes a short discussion on pertinent diagnostics, management, and other topics.
Surveys evaluating the program and teaching skill development were completed by 59 residents and 15 neurology educators who participated in BRAINs between 2022 and 2024.
Over 90% of trainees (54) agreed BRAINs sessions met the program’s objective (5 were neutral); 49 agreed it increased confidence in taking a neuro history (9 were neutral and 1 disagreed); 56 felt it boosted their confidence in doing a neuro exam (3 were neutral); and 56 said BRAINs is more effective than traditional lecture-based didactics (3 were neutral).
All the residents rated the material covered as appropriate for their level of training; 88% considered the 45-minute session length appropriate; and 98% had a favorable impression of the program as a whole.
When asked to identify the most helpful aspect of the program, 82% cited more patient exposure and 81% more bedside teaching.
All educators reported that the sessions were an effective way to practice near-peer teaching skills. Most (87%) felt the experience was more effective at accomplishing learning objectives than preparing and giving traditional didactic lectures, and 80% agreed it also gave them an opportunity to get to know their medical colleagues.
Use It or Lose It
Dr. Rajarajan noted that the program doesn’t require significant planning or extra staff, is not resource-intensive, and can be adapted to different services such as emergency departments and other learner populations.
But time will tell if the newfound confidence of those taking the program actually lasts.
“You have to keep using it,” he said. “You use it or lose it when comes to these skills.”
Commenting on the initiative, Denney Zimmerman, DO, Neurocritical Care Faculty, Blount Memorial Hospital, Maryville, Tennessee, and cochair of the AAN session featuring the study, called the program a good example of one way to counteract “neurophobia” and address the widespread neurologist shortage in the United States.
A 2019 AAN report showed that by 2025, almost every state in the United States will have a mismatch between the number of practicing neurologists and the demand from patients with neurologic conditions. The report offered several ways to address the shortage, including more neurology-focused training for internal medicine doctors during their residency.
“They’re usually on the front line, both in the hospital and in the clinics, and can help expedite patients who need to be seen by neurology sooner rather than later,” Dr. Zimmerman said.
Dr. Zimmerman noted that the study assessed how well participants perceived the program but not whether it improved their skills.
He pointed out that different groups may assess different diseases during their training session. “I think it’s important to ensure you’re hitting all the major topics.”
The study received funding from MGB Centers of Expertise Education Grant. Drs. Rajarajan and Zimmerman reported no relevant conflicts of interest.
A version of this article appeared on Medscape.com.
DENVER — , a new report suggested.
Bedside Rounding Alliance for Internal Medicine and Neurology Residents (BRAINs) moves training from the lecture hall to the bedside, offering instruction on obtaining a focused neurologic history and performing a focused neurologic physical exam for common neurologic symptoms.
Almost 100% of trainees surveyed gave the program a favorable rating, citing patient exposure and bedside training from neurology educators as keys to its success.
As internal medicine providers are often “the first to lay eyes” on patients with a neurology complaint, it’s important they “have a basic level of comfort” in addressing patients’ common questions and concerns, study author Prashanth Rajarajan, MD, PhD, a resident in the Department of Neurology at Brigham and Women’s Hospital, Boston, told this news organization.
The findings were presented at the 2024 annual meeting of the American Academy of Neurology.
Addressing ‘Neurophobia’
Neurology is often viewed by medical trainees as the most difficult subspecialty, Dr. Rajarajan said. Many have what he calls “neurophobia,” which he defines as “a discomfort with assessing and treating neurologic complaints.”
A survey at his institution showed 62% of internal medicine residents lacked the confidence to diagnose and treat neurologic diseases, he reported.
BRAINs is a structured neurology trainee-led, inpatient bedside teaching session for internal medicine residents, medical students, and others that aims to increase trainees’ confidence in assessing patients with common neurologic symptoms.
The program includes a biweekly 45-minute session. Most of the session is spent at the bedside and involves demonstrations and practice of a focused neurologic history and physical exam.
Participants receive feedback from educators, typically neurology residents or fellows in epilepsy, stroke, or some other neurology subspecialty. It also includes a short discussion on pertinent diagnostics, management, and other topics.
Surveys evaluating the program and teaching skill development were completed by 59 residents and 15 neurology educators who participated in BRAINs between 2022 and 2024.
Over 90% of trainees (54) agreed BRAINs sessions met the program’s objective (5 were neutral); 49 agreed it increased confidence in taking a neuro history (9 were neutral and 1 disagreed); 56 felt it boosted their confidence in doing a neuro exam (3 were neutral); and 56 said BRAINs is more effective than traditional lecture-based didactics (3 were neutral).
All the residents rated the material covered as appropriate for their level of training; 88% considered the 45-minute session length appropriate; and 98% had a favorable impression of the program as a whole.
When asked to identify the most helpful aspect of the program, 82% cited more patient exposure and 81% more bedside teaching.
All educators reported that the sessions were an effective way to practice near-peer teaching skills. Most (87%) felt the experience was more effective at accomplishing learning objectives than preparing and giving traditional didactic lectures, and 80% agreed it also gave them an opportunity to get to know their medical colleagues.
Use It or Lose It
Dr. Rajarajan noted that the program doesn’t require significant planning or extra staff, is not resource-intensive, and can be adapted to different services such as emergency departments and other learner populations.
But time will tell if the newfound confidence of those taking the program actually lasts.
“You have to keep using it,” he said. “You use it or lose it when comes to these skills.”
Commenting on the initiative, Denney Zimmerman, DO, Neurocritical Care Faculty, Blount Memorial Hospital, Maryville, Tennessee, and cochair of the AAN session featuring the study, called the program a good example of one way to counteract “neurophobia” and address the widespread neurologist shortage in the United States.
A 2019 AAN report showed that by 2025, almost every state in the United States will have a mismatch between the number of practicing neurologists and the demand from patients with neurologic conditions. The report offered several ways to address the shortage, including more neurology-focused training for internal medicine doctors during their residency.
“They’re usually on the front line, both in the hospital and in the clinics, and can help expedite patients who need to be seen by neurology sooner rather than later,” Dr. Zimmerman said.
Dr. Zimmerman noted that the study assessed how well participants perceived the program but not whether it improved their skills.
He pointed out that different groups may assess different diseases during their training session. “I think it’s important to ensure you’re hitting all the major topics.”
The study received funding from MGB Centers of Expertise Education Grant. Drs. Rajarajan and Zimmerman reported no relevant conflicts of interest.
A version of this article appeared on Medscape.com.
DENVER — , a new report suggested.
Bedside Rounding Alliance for Internal Medicine and Neurology Residents (BRAINs) moves training from the lecture hall to the bedside, offering instruction on obtaining a focused neurologic history and performing a focused neurologic physical exam for common neurologic symptoms.
Almost 100% of trainees surveyed gave the program a favorable rating, citing patient exposure and bedside training from neurology educators as keys to its success.
As internal medicine providers are often “the first to lay eyes” on patients with a neurology complaint, it’s important they “have a basic level of comfort” in addressing patients’ common questions and concerns, study author Prashanth Rajarajan, MD, PhD, a resident in the Department of Neurology at Brigham and Women’s Hospital, Boston, told this news organization.
The findings were presented at the 2024 annual meeting of the American Academy of Neurology.
Addressing ‘Neurophobia’
Neurology is often viewed by medical trainees as the most difficult subspecialty, Dr. Rajarajan said. Many have what he calls “neurophobia,” which he defines as “a discomfort with assessing and treating neurologic complaints.”
A survey at his institution showed 62% of internal medicine residents lacked the confidence to diagnose and treat neurologic diseases, he reported.
BRAINs is a structured neurology trainee-led, inpatient bedside teaching session for internal medicine residents, medical students, and others that aims to increase trainees’ confidence in assessing patients with common neurologic symptoms.
The program includes a biweekly 45-minute session. Most of the session is spent at the bedside and involves demonstrations and practice of a focused neurologic history and physical exam.
Participants receive feedback from educators, typically neurology residents or fellows in epilepsy, stroke, or some other neurology subspecialty. It also includes a short discussion on pertinent diagnostics, management, and other topics.
Surveys evaluating the program and teaching skill development were completed by 59 residents and 15 neurology educators who participated in BRAINs between 2022 and 2024.
Over 90% of trainees (54) agreed BRAINs sessions met the program’s objective (5 were neutral); 49 agreed it increased confidence in taking a neuro history (9 were neutral and 1 disagreed); 56 felt it boosted their confidence in doing a neuro exam (3 were neutral); and 56 said BRAINs is more effective than traditional lecture-based didactics (3 were neutral).
All the residents rated the material covered as appropriate for their level of training; 88% considered the 45-minute session length appropriate; and 98% had a favorable impression of the program as a whole.
When asked to identify the most helpful aspect of the program, 82% cited more patient exposure and 81% more bedside teaching.
All educators reported that the sessions were an effective way to practice near-peer teaching skills. Most (87%) felt the experience was more effective at accomplishing learning objectives than preparing and giving traditional didactic lectures, and 80% agreed it also gave them an opportunity to get to know their medical colleagues.
Use It or Lose It
Dr. Rajarajan noted that the program doesn’t require significant planning or extra staff, is not resource-intensive, and can be adapted to different services such as emergency departments and other learner populations.
But time will tell if the newfound confidence of those taking the program actually lasts.
“You have to keep using it,” he said. “You use it or lose it when comes to these skills.”
Commenting on the initiative, Denney Zimmerman, DO, Neurocritical Care Faculty, Blount Memorial Hospital, Maryville, Tennessee, and cochair of the AAN session featuring the study, called the program a good example of one way to counteract “neurophobia” and address the widespread neurologist shortage in the United States.
A 2019 AAN report showed that by 2025, almost every state in the United States will have a mismatch between the number of practicing neurologists and the demand from patients with neurologic conditions. The report offered several ways to address the shortage, including more neurology-focused training for internal medicine doctors during their residency.
“They’re usually on the front line, both in the hospital and in the clinics, and can help expedite patients who need to be seen by neurology sooner rather than later,” Dr. Zimmerman said.
Dr. Zimmerman noted that the study assessed how well participants perceived the program but not whether it improved their skills.
He pointed out that different groups may assess different diseases during their training session. “I think it’s important to ensure you’re hitting all the major topics.”
The study received funding from MGB Centers of Expertise Education Grant. Drs. Rajarajan and Zimmerman reported no relevant conflicts of interest.
A version of this article appeared on Medscape.com.
FROM AAN 2024
Risk for COVID-19 Infection in Patients With Vitiligo
To the Editor:
Vitiligo is a depigmentation disorder that results from the loss of melanocytes in the epidermis.1 The most widely accepted pathophysiology for melanocyte destruction in vitiligo is an autoimmune process involving dysregulated cytokine production and autoreactive T-cell activation.1 Individuals with cutaneous autoinflammatory conditions currently are vital patient populations warranting research, as their susceptibility to COVID-19 infection may differ from the general population. We previously found a small increased risk for COVID-19 infection in patients with psoriasis,2 which suggests that other dermatologic conditions also may impact COVID-19 risk. The risk for COVID-19 infection in patients with vitiligo remains largely unknown. In this retrospective cohort study, we investigated the risk for COVID-19 infection in patients with vitiligo compared with those without vitiligo utilizing claims data from the COVID-19 Research Database (https://covid19researchdatabase.org/).
Claims were evaluated for patients aged 3 years and older with a vitiligo diagnosis (International Classification of Diseases, Tenth Revision [ICD-10] code L80) that was made between January 1, 2016, and January 1, 2020. Individuals without a vitiligo diagnosis during the same period were placed (4:1 ratio) in the control group and were matched with study group patients for age and sex. All comorbidity variables and vitiligo diagnoses were extracted from ICD-10 codes that were given prior to a diagnosis of COVID-19. We then constructed multivariable logistic regression models adjusting for measured confounders to evaluate if vitiligo was associated with higher risk for COVID-19 infection after January 1, 2020.
The vitiligo and nonvitiligo cohorts included 40,363 and 161,452 patients, respectively (Table 1). Logistic regression analysis with adjustment for confounding variables, including high comorbid risk factors (Table 2) revealed that patients with a diagnosis of vitiligo had significantly increased odds of COVID-19 infection compared with patients without vitiligo (adjusted odds ratio [AOR], 1.47; 95% CI, 1.37-1.57; P<.001)(Table 3). Additionally, subgroup logistic analyses for sex, age, and exclusion of patients who were HIV positive revealed that females with vitiligo had higher odds of contracting COVID-19 than males with vitiligo (Table 3).
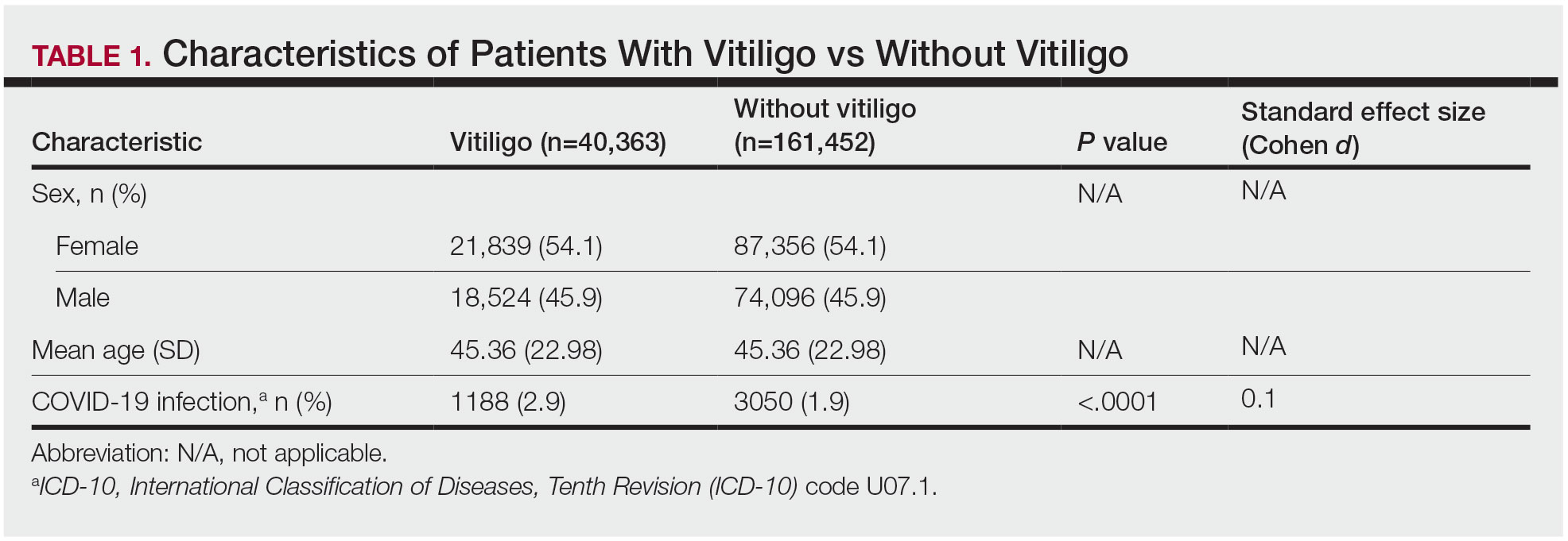
Our results showed that patients with vitiligo had a higher relative risk for contracting COVID-19 than individuals without vitiligo. It has been reported that the prevalence of COVID-19 is higher among patients with autoimmune diseases compared to the general population.3 Additionally, a handful of vitiligo patients are managed with immunosuppressive agents that may further weaken their immune response.1 Moreover, survey results from dermatologists managing vitiligo patients revealed that physicians were fairly comfortable prescribing immunosuppressants and encouraging in-office phototherapy during the COVID-19 pandemic.4 As a result, more patients may have been attending in-office visits for their phototherapy, which may have increased their risk for COVID-19. Although these factors play a role in COVID-19 infection rates, the underlying immune dysregulation in vitiligo in relation to COVID-19 remains unknown and should be further explored.
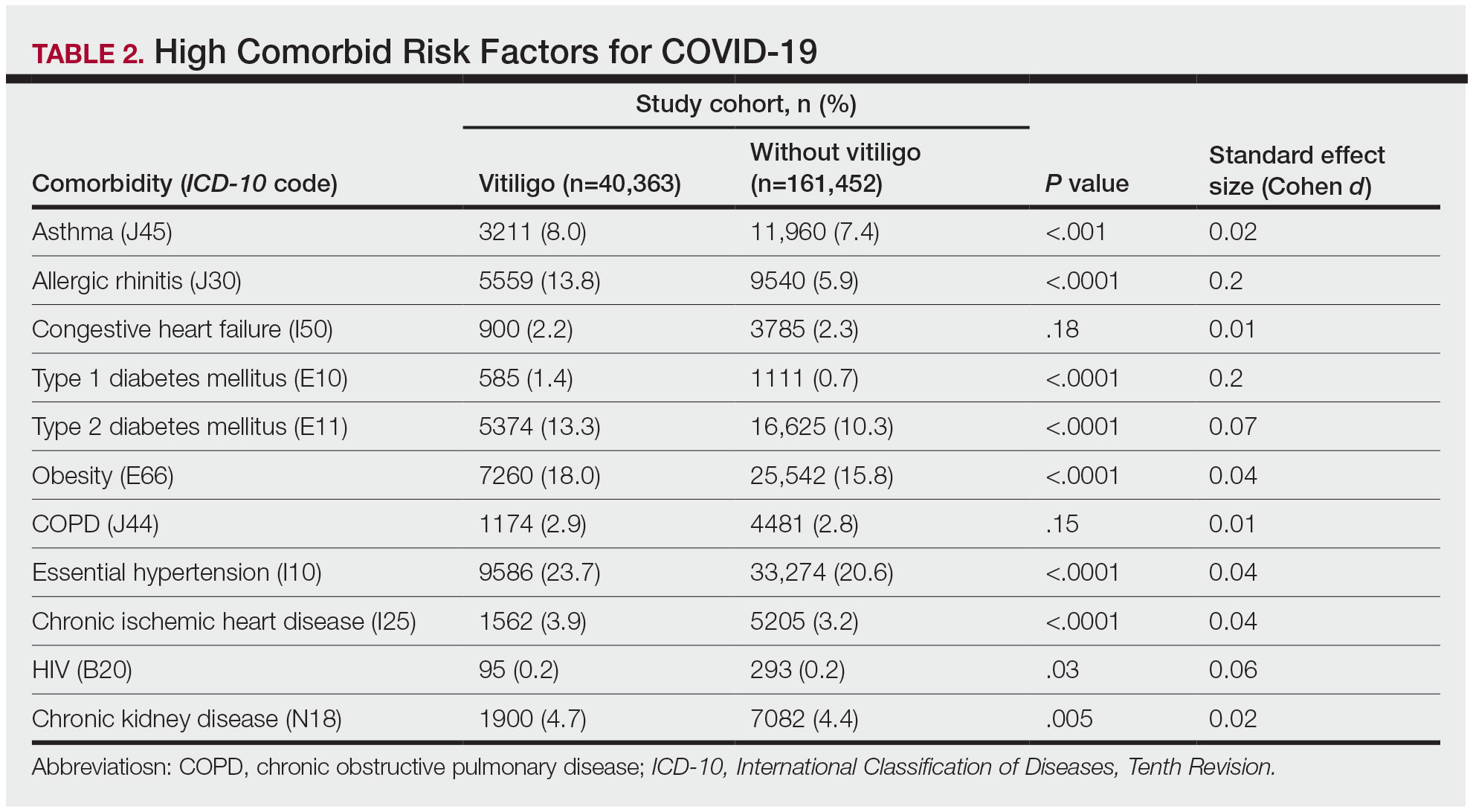
Our findings are limited by the use of ICD-10 codes, the inability to control for all potential confounding variables, the lack of data regarding the stage of vitiligo, and the absence of data for undiagnosed COVID-19 infections. In addition, patients with vitiligo may be more likely to seek care, potentially increasing their rates of COVID-19 testing. The inability to identify the stage of vitiligo during enrollment in the database may have altered our results, as individuals with active disease have increased levels of IFN-γ. Increased secretion of IFN-γ also potentially helps in the clearance of COVID-19 infection.1 Future studies should investigate this relationship via planned COVID-19 testing, identification of vitiligo stage, and controlling for other associated comorbidities.
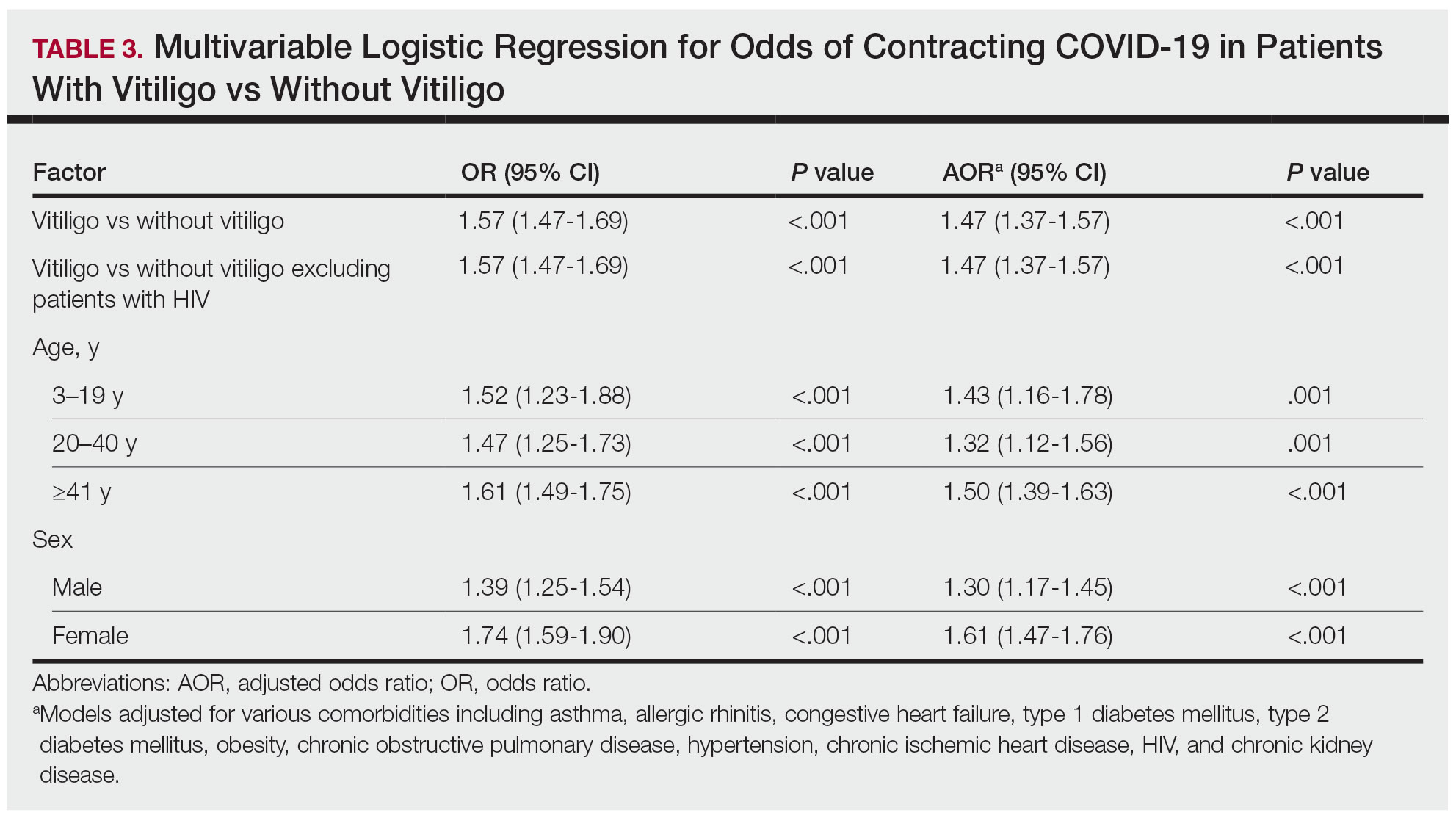
- Rashighi M, Harris JE. Vitiligo pathogenesis and emerging treatments. Dermatol Clin. 2017;35:257-265. doi:10.1016/j.det.2016.11.014
- Wu JJ, Liu J, Thatiparthi A, et al. The risk of COVID-19 in patients with psoriasis—a retrospective cohort study [published online September 20, 2022]. J Am Acad Dermatol. doi:10.1016/j.jaad.2022.07.040
- Zhong J, Shen G, Yang H, et al. COVID-19 in patients with rheumatic disease in Hubei province, China: a multicentre retrospective observational study. Lancet Rheumatol. 2020;2:E557-E564. doi:10.1016/S2665-9913(20)30227-7
- Chatterjee M, Das A. Management of vitiligo amidst the COVID-19 pandemic: a survey and resulting consensus. Indian J Dermatol. 2021;66:479-483. doi:10.4103/ijd.ijd_859_20
To the Editor:
Vitiligo is a depigmentation disorder that results from the loss of melanocytes in the epidermis.1 The most widely accepted pathophysiology for melanocyte destruction in vitiligo is an autoimmune process involving dysregulated cytokine production and autoreactive T-cell activation.1 Individuals with cutaneous autoinflammatory conditions currently are vital patient populations warranting research, as their susceptibility to COVID-19 infection may differ from the general population. We previously found a small increased risk for COVID-19 infection in patients with psoriasis,2 which suggests that other dermatologic conditions also may impact COVID-19 risk. The risk for COVID-19 infection in patients with vitiligo remains largely unknown. In this retrospective cohort study, we investigated the risk for COVID-19 infection in patients with vitiligo compared with those without vitiligo utilizing claims data from the COVID-19 Research Database (https://covid19researchdatabase.org/).
Claims were evaluated for patients aged 3 years and older with a vitiligo diagnosis (International Classification of Diseases, Tenth Revision [ICD-10] code L80) that was made between January 1, 2016, and January 1, 2020. Individuals without a vitiligo diagnosis during the same period were placed (4:1 ratio) in the control group and were matched with study group patients for age and sex. All comorbidity variables and vitiligo diagnoses were extracted from ICD-10 codes that were given prior to a diagnosis of COVID-19. We then constructed multivariable logistic regression models adjusting for measured confounders to evaluate if vitiligo was associated with higher risk for COVID-19 infection after January 1, 2020.
The vitiligo and nonvitiligo cohorts included 40,363 and 161,452 patients, respectively (Table 1). Logistic regression analysis with adjustment for confounding variables, including high comorbid risk factors (Table 2) revealed that patients with a diagnosis of vitiligo had significantly increased odds of COVID-19 infection compared with patients without vitiligo (adjusted odds ratio [AOR], 1.47; 95% CI, 1.37-1.57; P<.001)(Table 3). Additionally, subgroup logistic analyses for sex, age, and exclusion of patients who were HIV positive revealed that females with vitiligo had higher odds of contracting COVID-19 than males with vitiligo (Table 3).

Our results showed that patients with vitiligo had a higher relative risk for contracting COVID-19 than individuals without vitiligo. It has been reported that the prevalence of COVID-19 is higher among patients with autoimmune diseases compared to the general population.3 Additionally, a handful of vitiligo patients are managed with immunosuppressive agents that may further weaken their immune response.1 Moreover, survey results from dermatologists managing vitiligo patients revealed that physicians were fairly comfortable prescribing immunosuppressants and encouraging in-office phototherapy during the COVID-19 pandemic.4 As a result, more patients may have been attending in-office visits for their phototherapy, which may have increased their risk for COVID-19. Although these factors play a role in COVID-19 infection rates, the underlying immune dysregulation in vitiligo in relation to COVID-19 remains unknown and should be further explored.

Our findings are limited by the use of ICD-10 codes, the inability to control for all potential confounding variables, the lack of data regarding the stage of vitiligo, and the absence of data for undiagnosed COVID-19 infections. In addition, patients with vitiligo may be more likely to seek care, potentially increasing their rates of COVID-19 testing. The inability to identify the stage of vitiligo during enrollment in the database may have altered our results, as individuals with active disease have increased levels of IFN-γ. Increased secretion of IFN-γ also potentially helps in the clearance of COVID-19 infection.1 Future studies should investigate this relationship via planned COVID-19 testing, identification of vitiligo stage, and controlling for other associated comorbidities.

To the Editor:
Vitiligo is a depigmentation disorder that results from the loss of melanocytes in the epidermis.1 The most widely accepted pathophysiology for melanocyte destruction in vitiligo is an autoimmune process involving dysregulated cytokine production and autoreactive T-cell activation.1 Individuals with cutaneous autoinflammatory conditions currently are vital patient populations warranting research, as their susceptibility to COVID-19 infection may differ from the general population. We previously found a small increased risk for COVID-19 infection in patients with psoriasis,2 which suggests that other dermatologic conditions also may impact COVID-19 risk. The risk for COVID-19 infection in patients with vitiligo remains largely unknown. In this retrospective cohort study, we investigated the risk for COVID-19 infection in patients with vitiligo compared with those without vitiligo utilizing claims data from the COVID-19 Research Database (https://covid19researchdatabase.org/).
Claims were evaluated for patients aged 3 years and older with a vitiligo diagnosis (International Classification of Diseases, Tenth Revision [ICD-10] code L80) that was made between January 1, 2016, and January 1, 2020. Individuals without a vitiligo diagnosis during the same period were placed (4:1 ratio) in the control group and were matched with study group patients for age and sex. All comorbidity variables and vitiligo diagnoses were extracted from ICD-10 codes that were given prior to a diagnosis of COVID-19. We then constructed multivariable logistic regression models adjusting for measured confounders to evaluate if vitiligo was associated with higher risk for COVID-19 infection after January 1, 2020.
The vitiligo and nonvitiligo cohorts included 40,363 and 161,452 patients, respectively (Table 1). Logistic regression analysis with adjustment for confounding variables, including high comorbid risk factors (Table 2) revealed that patients with a diagnosis of vitiligo had significantly increased odds of COVID-19 infection compared with patients without vitiligo (adjusted odds ratio [AOR], 1.47; 95% CI, 1.37-1.57; P<.001)(Table 3). Additionally, subgroup logistic analyses for sex, age, and exclusion of patients who were HIV positive revealed that females with vitiligo had higher odds of contracting COVID-19 than males with vitiligo (Table 3).

Our results showed that patients with vitiligo had a higher relative risk for contracting COVID-19 than individuals without vitiligo. It has been reported that the prevalence of COVID-19 is higher among patients with autoimmune diseases compared to the general population.3 Additionally, a handful of vitiligo patients are managed with immunosuppressive agents that may further weaken their immune response.1 Moreover, survey results from dermatologists managing vitiligo patients revealed that physicians were fairly comfortable prescribing immunosuppressants and encouraging in-office phototherapy during the COVID-19 pandemic.4 As a result, more patients may have been attending in-office visits for their phototherapy, which may have increased their risk for COVID-19. Although these factors play a role in COVID-19 infection rates, the underlying immune dysregulation in vitiligo in relation to COVID-19 remains unknown and should be further explored.

Our findings are limited by the use of ICD-10 codes, the inability to control for all potential confounding variables, the lack of data regarding the stage of vitiligo, and the absence of data for undiagnosed COVID-19 infections. In addition, patients with vitiligo may be more likely to seek care, potentially increasing their rates of COVID-19 testing. The inability to identify the stage of vitiligo during enrollment in the database may have altered our results, as individuals with active disease have increased levels of IFN-γ. Increased secretion of IFN-γ also potentially helps in the clearance of COVID-19 infection.1 Future studies should investigate this relationship via planned COVID-19 testing, identification of vitiligo stage, and controlling for other associated comorbidities.

- Rashighi M, Harris JE. Vitiligo pathogenesis and emerging treatments. Dermatol Clin. 2017;35:257-265. doi:10.1016/j.det.2016.11.014
- Wu JJ, Liu J, Thatiparthi A, et al. The risk of COVID-19 in patients with psoriasis—a retrospective cohort study [published online September 20, 2022]. J Am Acad Dermatol. doi:10.1016/j.jaad.2022.07.040
- Zhong J, Shen G, Yang H, et al. COVID-19 in patients with rheumatic disease in Hubei province, China: a multicentre retrospective observational study. Lancet Rheumatol. 2020;2:E557-E564. doi:10.1016/S2665-9913(20)30227-7
- Chatterjee M, Das A. Management of vitiligo amidst the COVID-19 pandemic: a survey and resulting consensus. Indian J Dermatol. 2021;66:479-483. doi:10.4103/ijd.ijd_859_20
- Rashighi M, Harris JE. Vitiligo pathogenesis and emerging treatments. Dermatol Clin. 2017;35:257-265. doi:10.1016/j.det.2016.11.014
- Wu JJ, Liu J, Thatiparthi A, et al. The risk of COVID-19 in patients with psoriasis—a retrospective cohort study [published online September 20, 2022]. J Am Acad Dermatol. doi:10.1016/j.jaad.2022.07.040
- Zhong J, Shen G, Yang H, et al. COVID-19 in patients with rheumatic disease in Hubei province, China: a multicentre retrospective observational study. Lancet Rheumatol. 2020;2:E557-E564. doi:10.1016/S2665-9913(20)30227-7
- Chatterjee M, Das A. Management of vitiligo amidst the COVID-19 pandemic: a survey and resulting consensus. Indian J Dermatol. 2021;66:479-483. doi:10.4103/ijd.ijd_859_20
Practice Points
- The underlying autoimmune process in vitiligo can result in various changes to the immune system.
- A diagnosis of vitiligo may alter the body’s immune response to COVID-19 infection.
Few Cancer Survivors Meet ACS Nutrition, Exercise Guidelines
TOPLINE:
METHODOLOGY:
- The ACS has published nutrition and exercise guidelines for cancer survivors, which include recommendations to maintain a healthy weight and diet, cut out alcohol, and participate in regular physical activities. Engaging in these behaviors is associated with longer survival among cancer survivors, but whether survivors follow these nutrition and activity recommendations has not been systematically tracked.
- Researchers evaluated data on 10,020 individuals (mean age, 64.2 years) who had completed cancer treatment. Data came from the Behavioral Risk Factor Surveillance System telephone-based survey administered in 2017, 2019, and 2021, which represents 2.7 million cancer survivors.
- The researchers estimated survivors’ adherence to guidelines across four domains: Weight, physical activity, fruit and vegetable consumption, and alcohol intake. Factors associated with adherence were also evaluated.
- Overall, 9,121 survivors (91%) completed questionnaires for all four domains.
TAKEAWAY:
Only 4% of patients (365 of 9121) followed ACS guidelines in all four categories.
When assessing adherence to each category, the researchers found that 72% of cancer survivors reported engaging in recommended levels of physical activity, 68% maintained a nonobese weight, 50% said they did not consume alcohol, and 12% said they consumed recommended quantities of fruits and vegetables.
Compared with people in the general population, cancer survivors generally engaged in fewer healthy behaviors than those who had never been diagnosed with cancer.
The authors identified certain factors associated with greater guideline adherence, including female sex, older age, Black (vs White) race, and higher education level (college graduate).
IN PRACTICE:
This study highlights a potential “gap between published guidelines regarding behavioral modifications for cancer survivors and uptake of these behaviors,” the authors wrote, adding that “it is essential for oncologists and general internists to improve widespread and systematic counseling on these guidelines to improve uptake of healthy behaviors in this vulnerable patient population.”
SOURCE:
This work, led by Carter Baughman, MD, from the Division of Internal Medicine at Beth Israel Deaconess Medical Center, Boston, Massachusetts, was published online in JAMA Oncology.
LIMITATIONS:
The authors reported several study limitations, most notably that self-reported data may introduce biases.
DISCLOSURES:
The study funding source was not reported. One author received grants from the US Highbush Blueberry Council outside the submitted work. No other disclosures were reported.
A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- The ACS has published nutrition and exercise guidelines for cancer survivors, which include recommendations to maintain a healthy weight and diet, cut out alcohol, and participate in regular physical activities. Engaging in these behaviors is associated with longer survival among cancer survivors, but whether survivors follow these nutrition and activity recommendations has not been systematically tracked.
- Researchers evaluated data on 10,020 individuals (mean age, 64.2 years) who had completed cancer treatment. Data came from the Behavioral Risk Factor Surveillance System telephone-based survey administered in 2017, 2019, and 2021, which represents 2.7 million cancer survivors.
- The researchers estimated survivors’ adherence to guidelines across four domains: Weight, physical activity, fruit and vegetable consumption, and alcohol intake. Factors associated with adherence were also evaluated.
- Overall, 9,121 survivors (91%) completed questionnaires for all four domains.
TAKEAWAY:
Only 4% of patients (365 of 9121) followed ACS guidelines in all four categories.
When assessing adherence to each category, the researchers found that 72% of cancer survivors reported engaging in recommended levels of physical activity, 68% maintained a nonobese weight, 50% said they did not consume alcohol, and 12% said they consumed recommended quantities of fruits and vegetables.
Compared with people in the general population, cancer survivors generally engaged in fewer healthy behaviors than those who had never been diagnosed with cancer.
The authors identified certain factors associated with greater guideline adherence, including female sex, older age, Black (vs White) race, and higher education level (college graduate).
IN PRACTICE:
This study highlights a potential “gap between published guidelines regarding behavioral modifications for cancer survivors and uptake of these behaviors,” the authors wrote, adding that “it is essential for oncologists and general internists to improve widespread and systematic counseling on these guidelines to improve uptake of healthy behaviors in this vulnerable patient population.”
SOURCE:
This work, led by Carter Baughman, MD, from the Division of Internal Medicine at Beth Israel Deaconess Medical Center, Boston, Massachusetts, was published online in JAMA Oncology.
LIMITATIONS:
The authors reported several study limitations, most notably that self-reported data may introduce biases.
DISCLOSURES:
The study funding source was not reported. One author received grants from the US Highbush Blueberry Council outside the submitted work. No other disclosures were reported.
A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- The ACS has published nutrition and exercise guidelines for cancer survivors, which include recommendations to maintain a healthy weight and diet, cut out alcohol, and participate in regular physical activities. Engaging in these behaviors is associated with longer survival among cancer survivors, but whether survivors follow these nutrition and activity recommendations has not been systematically tracked.
- Researchers evaluated data on 10,020 individuals (mean age, 64.2 years) who had completed cancer treatment. Data came from the Behavioral Risk Factor Surveillance System telephone-based survey administered in 2017, 2019, and 2021, which represents 2.7 million cancer survivors.
- The researchers estimated survivors’ adherence to guidelines across four domains: Weight, physical activity, fruit and vegetable consumption, and alcohol intake. Factors associated with adherence were also evaluated.
- Overall, 9,121 survivors (91%) completed questionnaires for all four domains.
TAKEAWAY:
Only 4% of patients (365 of 9121) followed ACS guidelines in all four categories.
When assessing adherence to each category, the researchers found that 72% of cancer survivors reported engaging in recommended levels of physical activity, 68% maintained a nonobese weight, 50% said they did not consume alcohol, and 12% said they consumed recommended quantities of fruits and vegetables.
Compared with people in the general population, cancer survivors generally engaged in fewer healthy behaviors than those who had never been diagnosed with cancer.
The authors identified certain factors associated with greater guideline adherence, including female sex, older age, Black (vs White) race, and higher education level (college graduate).
IN PRACTICE:
This study highlights a potential “gap between published guidelines regarding behavioral modifications for cancer survivors and uptake of these behaviors,” the authors wrote, adding that “it is essential for oncologists and general internists to improve widespread and systematic counseling on these guidelines to improve uptake of healthy behaviors in this vulnerable patient population.”
SOURCE:
This work, led by Carter Baughman, MD, from the Division of Internal Medicine at Beth Israel Deaconess Medical Center, Boston, Massachusetts, was published online in JAMA Oncology.
LIMITATIONS:
The authors reported several study limitations, most notably that self-reported data may introduce biases.
DISCLOSURES:
The study funding source was not reported. One author received grants from the US Highbush Blueberry Council outside the submitted work. No other disclosures were reported.
A version of this article appeared on Medscape.com.