User login
The Future of Polycythemia Vera

There are several new therapies on the horizon for polycythemia vera. What is the potential impact of these treatments coming to market?
Dr. Richard: There are a number of emerging therapies for polycythemia vera (PV), such as PTG-300, idasanutlin, and givinostat. PTG-300, or rusfertide, is a hepcidin mimetic that works by regulating iron metabolism and potentially controlling erythropoiesis, limiting the need for phlebotomy. Idasanutlin, a selective MDM2 inhibitor, targets p53 activity. Even though this drug is early in its development, everyone who treats patients with cancer has been hoping for a drug that works through p53. If it is effective here, who knows where else it could be effective across various other conditions.
Givinostat is well along the development pathway in advanced trials. This drug shows promise in modulating gene expression and reducing the inflammation and fibrosis associated with PV, potentially improving patient outcomes and quality of life. Everyone is hopeful that givinostat could show some effect on disease control and potentially an effect on the myeloproliferative clone. However, rigorous clinical trials and further research are necessary to validate their efficacy, safety profiles, and long-term impacts on patients with PV.
Now, with the approval of peginterferon, the next step is going to be to see how effective it will be and what the adverse events might be. I think we will be getting more data as it starts to be used more. My prediction is that there will be a slow uptake, largely because many older physicians such as myself remember the significant side effects from interferon in the past. Despite being an FDA-approved treatment, it remains an emerging therapy, particularly in the United States. Its adoption and efficacy will become clearer as time progresses.
Another promising drug early in its development is bomedemstat, which functions through a different mechanism as a deacetylase. While the potential effect of histone deacetylase drugs on patient treatment outcomes remains uncertain this year, there might be significant data—either positive or negative—that accelerate the progress of these drugs in their developmental trajectory.
We know that ruxolitinib can be used effectively for patients once they fail hydroxyurea. And now there has been the development of other JAK2 inhibitors that are approved for myelofibrosis. I am not quite sure how they can be evaluated in PV, since we are talking about relatively small numbers of patients, but they do seem to have some slight differences that may be significant and could be used in this space.
Those are the main therapies that I will have my eye on this year.
What is the potential significance of an accelerated dosing schedule for BESREMi (ropeginterferon-alfa-2b-njft), which is being investigated in the ECLIPSE PV phase 3b clinical trial?
Dr. Richard: The potential significance of an accelerated dosing schedule for BESREMi, as investigated in the ECLIPSE PV phase 3b clinical trial, lies in its capacity to enhance treatment efficacy and outcomes for patients with PV. I am incredibly pleased that it is being done as a trial, partly because a lot of people assume that once a phase 3 study is complete and a drug receives FDA approval, everything is finished and done, and we will move on to the next thing. I really appreciate it when phase 3b or 4 studies are performed, and the data get collected and published.
This study is going to follow a group of patients closely for adverse events and for the JAK2 signal. By administering BESREMi at an accelerated pace, researchers can evaluate its ability to better control hematocrit levels and symptoms associated with PV. In addition, an accelerated dosing schedule could potentially offer patients more efficient symptom management and disease control, leading to improved quality of life and reduced complications associated with PV. I believe that findings from this trial could thus pave the way for optimized treatment strategies and better outcomes for individuals living with PV.
What should future trials focus on to help improve prognosis and survival for patients with PV?
Dr. Richard: We are starting to move increasingly into finding better therapies for patients with PV, and I’ll add in essential thrombocytosis, which are based on informed prognostication. I would love to see studies that just pull out the patients at the highest risk, where the survival is down around 5 years—those are small numbers of patients. To conduct a study like that is exceedingly difficult to do. We are seeing increased consortiums of myeloproliferative neoplasm physicians. Europe has always been particularly good at this. The United States is getting better at it, so it is possible that a trial like that could be pulled together, where centers put in 1 or 2 patients at a time.
Future trials aimed at improving prognosis and survival for PV should prioritize several critical areas. First, there is a need for comprehensive studies to better understand the molecular mechanisms underlying PV pathogenesis, including the JAK2 mutation and its downstream effects. Exploring new therapeutic implications and improve long-term outcomes. Additionally, identifying reliable biomarkers for disease progression and treatment response can facilitate early intervention and personalized treatment approaches. Finally, trials should focus on assessing the impact of treatment on quality of life and addressing the unique needs of patients with PV to optimize overall prognosis and survival.
I have always held hope that the Veterans Administration could serve as a platform for conducting some of these studies, given that we possess the largest healthcare system in the country. Whether we participate in larger studies or conduct our research internally, this is something I have long envisioned.

There are several new therapies on the horizon for polycythemia vera. What is the potential impact of these treatments coming to market?
Dr. Richard: There are a number of emerging therapies for polycythemia vera (PV), such as PTG-300, idasanutlin, and givinostat. PTG-300, or rusfertide, is a hepcidin mimetic that works by regulating iron metabolism and potentially controlling erythropoiesis, limiting the need for phlebotomy. Idasanutlin, a selective MDM2 inhibitor, targets p53 activity. Even though this drug is early in its development, everyone who treats patients with cancer has been hoping for a drug that works through p53. If it is effective here, who knows where else it could be effective across various other conditions.
Givinostat is well along the development pathway in advanced trials. This drug shows promise in modulating gene expression and reducing the inflammation and fibrosis associated with PV, potentially improving patient outcomes and quality of life. Everyone is hopeful that givinostat could show some effect on disease control and potentially an effect on the myeloproliferative clone. However, rigorous clinical trials and further research are necessary to validate their efficacy, safety profiles, and long-term impacts on patients with PV.
Now, with the approval of peginterferon, the next step is going to be to see how effective it will be and what the adverse events might be. I think we will be getting more data as it starts to be used more. My prediction is that there will be a slow uptake, largely because many older physicians such as myself remember the significant side effects from interferon in the past. Despite being an FDA-approved treatment, it remains an emerging therapy, particularly in the United States. Its adoption and efficacy will become clearer as time progresses.
Another promising drug early in its development is bomedemstat, which functions through a different mechanism as a deacetylase. While the potential effect of histone deacetylase drugs on patient treatment outcomes remains uncertain this year, there might be significant data—either positive or negative—that accelerate the progress of these drugs in their developmental trajectory.
We know that ruxolitinib can be used effectively for patients once they fail hydroxyurea. And now there has been the development of other JAK2 inhibitors that are approved for myelofibrosis. I am not quite sure how they can be evaluated in PV, since we are talking about relatively small numbers of patients, but they do seem to have some slight differences that may be significant and could be used in this space.
Those are the main therapies that I will have my eye on this year.
What is the potential significance of an accelerated dosing schedule for BESREMi (ropeginterferon-alfa-2b-njft), which is being investigated in the ECLIPSE PV phase 3b clinical trial?
Dr. Richard: The potential significance of an accelerated dosing schedule for BESREMi, as investigated in the ECLIPSE PV phase 3b clinical trial, lies in its capacity to enhance treatment efficacy and outcomes for patients with PV. I am incredibly pleased that it is being done as a trial, partly because a lot of people assume that once a phase 3 study is complete and a drug receives FDA approval, everything is finished and done, and we will move on to the next thing. I really appreciate it when phase 3b or 4 studies are performed, and the data get collected and published.
This study is going to follow a group of patients closely for adverse events and for the JAK2 signal. By administering BESREMi at an accelerated pace, researchers can evaluate its ability to better control hematocrit levels and symptoms associated with PV. In addition, an accelerated dosing schedule could potentially offer patients more efficient symptom management and disease control, leading to improved quality of life and reduced complications associated with PV. I believe that findings from this trial could thus pave the way for optimized treatment strategies and better outcomes for individuals living with PV.
What should future trials focus on to help improve prognosis and survival for patients with PV?
Dr. Richard: We are starting to move increasingly into finding better therapies for patients with PV, and I’ll add in essential thrombocytosis, which are based on informed prognostication. I would love to see studies that just pull out the patients at the highest risk, where the survival is down around 5 years—those are small numbers of patients. To conduct a study like that is exceedingly difficult to do. We are seeing increased consortiums of myeloproliferative neoplasm physicians. Europe has always been particularly good at this. The United States is getting better at it, so it is possible that a trial like that could be pulled together, where centers put in 1 or 2 patients at a time.
Future trials aimed at improving prognosis and survival for PV should prioritize several critical areas. First, there is a need for comprehensive studies to better understand the molecular mechanisms underlying PV pathogenesis, including the JAK2 mutation and its downstream effects. Exploring new therapeutic implications and improve long-term outcomes. Additionally, identifying reliable biomarkers for disease progression and treatment response can facilitate early intervention and personalized treatment approaches. Finally, trials should focus on assessing the impact of treatment on quality of life and addressing the unique needs of patients with PV to optimize overall prognosis and survival.
I have always held hope that the Veterans Administration could serve as a platform for conducting some of these studies, given that we possess the largest healthcare system in the country. Whether we participate in larger studies or conduct our research internally, this is something I have long envisioned.

There are several new therapies on the horizon for polycythemia vera. What is the potential impact of these treatments coming to market?
Dr. Richard: There are a number of emerging therapies for polycythemia vera (PV), such as PTG-300, idasanutlin, and givinostat. PTG-300, or rusfertide, is a hepcidin mimetic that works by regulating iron metabolism and potentially controlling erythropoiesis, limiting the need for phlebotomy. Idasanutlin, a selective MDM2 inhibitor, targets p53 activity. Even though this drug is early in its development, everyone who treats patients with cancer has been hoping for a drug that works through p53. If it is effective here, who knows where else it could be effective across various other conditions.
Givinostat is well along the development pathway in advanced trials. This drug shows promise in modulating gene expression and reducing the inflammation and fibrosis associated with PV, potentially improving patient outcomes and quality of life. Everyone is hopeful that givinostat could show some effect on disease control and potentially an effect on the myeloproliferative clone. However, rigorous clinical trials and further research are necessary to validate their efficacy, safety profiles, and long-term impacts on patients with PV.
Now, with the approval of peginterferon, the next step is going to be to see how effective it will be and what the adverse events might be. I think we will be getting more data as it starts to be used more. My prediction is that there will be a slow uptake, largely because many older physicians such as myself remember the significant side effects from interferon in the past. Despite being an FDA-approved treatment, it remains an emerging therapy, particularly in the United States. Its adoption and efficacy will become clearer as time progresses.
Another promising drug early in its development is bomedemstat, which functions through a different mechanism as a deacetylase. While the potential effect of histone deacetylase drugs on patient treatment outcomes remains uncertain this year, there might be significant data—either positive or negative—that accelerate the progress of these drugs in their developmental trajectory.
We know that ruxolitinib can be used effectively for patients once they fail hydroxyurea. And now there has been the development of other JAK2 inhibitors that are approved for myelofibrosis. I am not quite sure how they can be evaluated in PV, since we are talking about relatively small numbers of patients, but they do seem to have some slight differences that may be significant and could be used in this space.
Those are the main therapies that I will have my eye on this year.
What is the potential significance of an accelerated dosing schedule for BESREMi (ropeginterferon-alfa-2b-njft), which is being investigated in the ECLIPSE PV phase 3b clinical trial?
Dr. Richard: The potential significance of an accelerated dosing schedule for BESREMi, as investigated in the ECLIPSE PV phase 3b clinical trial, lies in its capacity to enhance treatment efficacy and outcomes for patients with PV. I am incredibly pleased that it is being done as a trial, partly because a lot of people assume that once a phase 3 study is complete and a drug receives FDA approval, everything is finished and done, and we will move on to the next thing. I really appreciate it when phase 3b or 4 studies are performed, and the data get collected and published.
This study is going to follow a group of patients closely for adverse events and for the JAK2 signal. By administering BESREMi at an accelerated pace, researchers can evaluate its ability to better control hematocrit levels and symptoms associated with PV. In addition, an accelerated dosing schedule could potentially offer patients more efficient symptom management and disease control, leading to improved quality of life and reduced complications associated with PV. I believe that findings from this trial could thus pave the way for optimized treatment strategies and better outcomes for individuals living with PV.
What should future trials focus on to help improve prognosis and survival for patients with PV?
Dr. Richard: We are starting to move increasingly into finding better therapies for patients with PV, and I’ll add in essential thrombocytosis, which are based on informed prognostication. I would love to see studies that just pull out the patients at the highest risk, where the survival is down around 5 years—those are small numbers of patients. To conduct a study like that is exceedingly difficult to do. We are seeing increased consortiums of myeloproliferative neoplasm physicians. Europe has always been particularly good at this. The United States is getting better at it, so it is possible that a trial like that could be pulled together, where centers put in 1 or 2 patients at a time.
Future trials aimed at improving prognosis and survival for PV should prioritize several critical areas. First, there is a need for comprehensive studies to better understand the molecular mechanisms underlying PV pathogenesis, including the JAK2 mutation and its downstream effects. Exploring new therapeutic implications and improve long-term outcomes. Additionally, identifying reliable biomarkers for disease progression and treatment response can facilitate early intervention and personalized treatment approaches. Finally, trials should focus on assessing the impact of treatment on quality of life and addressing the unique needs of patients with PV to optimize overall prognosis and survival.
I have always held hope that the Veterans Administration could serve as a platform for conducting some of these studies, given that we possess the largest healthcare system in the country. Whether we participate in larger studies or conduct our research internally, this is something I have long envisioned.
JAK Inhibitors for Vitiligo: Response Continues Over Time
SAN DIEGO — according to presentations at a late-breaking session at the annual meeting of the American Academy of Dermatology (AAD).
In one, the addition of narrow-band ultraviolet-B (NB-UVB) light therapy to ritlecitinib appears more effective than ritlecitinib alone. In the other study, the effectiveness of upadacitinib appears to improve over time.
Based on the ritlecitinib data, “if you have phototherapy in your office, it might be good to couple it with ritlecitinib for vitiligo patients,” said Emma Guttman-Yassky, MD, PhD, chair of the Department of Dermatology, Icahn School of Medicine at Mount Sinai, New York City, who presented the findings.
However, because of the relatively small numbers in the extension study, Dr. Guttman-Yassky characterized the evidence as preliminary and in need of further investigation.
For vitiligo, the only approved JAK inhibitor is ruxolitinib, 1.5%, in a cream formulation. In June, ritlecitinib (Litfulo) was approved by the Food and Drug Administration (FDA) for alopecia areata. Phototherapy, which has been used for decades in the treatment of vitiligo, has an established efficacy and safety profile as a stand-alone vitiligo treatment. Upadacitinib has numerous indications for inflammatory diseases, such as rheumatoid arthritis, and was granted FDA approval for atopic dermatitis in 2022.
NB-UVB Arm Added in Ritlecitinib Extension
The ritlecitinib study population was drawn from patients with non-segmental vitiligo who initially participated in a 24-week dose-ranging period of a phase 2b trial published last year. In that study, 364 patients were randomized to doses of once-daily ritlecitinib ranging from 10 to 50 mg with or without a 4-week loading regimen. Higher doses were generally associated with greater efficacy on the primary endpoint of facial vitiligo area scoring index (F-VASI) but not with a greater risk for adverse events.
In the 24-week extension study, 187 patients received a 4-week loading regimen of 200-mg ritlecitinib daily followed by 50 mg of daily ritlecitinib for the remaining 20 weeks. Another 43 patients were randomized to one of two arms: The same 4-week loading regimen of 200-mg ritlecitinib daily followed by 50 mg of daily ritlecitinib or to 50-mg daily ritlecitinib without a loading dose but combined with NB-UVB delivered twice per week.
Important to interpretation of results, there was an additional twist. Patients in the randomized arm who had < 10% improvement in the total vitiligo area severity index (T-VASI) at week 12 of the extension were discontinued from the study.
The endpoints considered when comparing ritlecitinib with or without NB-UVB at the end of the extension study were F-VASI, T-VASI, patient global impression of change, and adverse events. Responses were assessed on the basis of both observed and last observation carried forward (LOCF).
Of the 43 people, who were randomized in the extension study, nine (21%) had < 10% improvement in T-VASI and were therefore discontinued from the study.
At the end of 24 weeks, both groups had a substantial response to their assigned therapy, but the addition of NB-UVB increased rates of response, although not always at a level of statistical significance, according to Dr. Guttman-Yassky.
For the percent improvement in F-VASI, specifically, the increase did not reach significance on the basis of LOCF (57.9% vs 51.5%; P = .158) but was highly significant on the basis of observed responses (69.6% vs 55.1%; P = .009). For T-VASI, differences for adjunctive NB-UVB over monotherapy did not reach significance for either observed or LOCF responses, but it was significant for observed responses in a patient global impression of change.
Small Numbers Limit Strength of Ritlecitinib, NB-UVB Evidence
However, Dr. Guttman-Yassky said it is important “to pay attention to the sample sizes” when noting the lack of significance.
The combination appeared safe, and there were no side effects associated with the addition of twice-weekly NB-UVB to ritlecitinib.
She acknowledged that the design of this analysis was “complicated” and that the number of randomized patients was small. She suggested the findings support the potential for benefit from the combination of a JAK inhibitor and NB-UVB, both of which have shown efficacy as monotherapy in previous studies. She indicated that a trial of this combination is reasonable while awaiting a more definitive study.
One of the questions that might be posed in a larger study is the timing of NB-UVB, such as whether it is best reserved for those with inadequate early response to a JAK inhibitor or if optimal results are achieved when a JAK inhibitor and NB-UVB are initiated simultaneously.
Upadacitinib Monotherapy Results
One rationale for initiating therapy with the combination of a JAK inhibitor and NB-UVB is the potential for a more rapid response, but extended results from a second phase 2b study with a different oral JAK inhibitor, upadacitinib, suggested responses on JAK inhibitor monotherapy improve steadily over time.
“The overall efficacy continued to improve without reaching a plateau at 1 year,” reported Thierry Passeron, MD, PhD, professor and chair, Department of Dermatology, Université Côte d’Azur, Nice, France. He spoke at the same AAD late-breaking session as Dr. Guttman-Yassky.
The 24-week dose-ranging data from the upadacitinib trial were previously reported at the 2023 annual meeting of the European Association of Dermatology and Venereology. In the placebo-controlled portion, which randomized 185 patients with extensive non-segmental vitiligo to 6 mg, 11 mg, or 22 mg, the two higher doses were significantly more effective than placebo.
In the extension, patients in the placebo group were randomized to 11 mg or 22 mg, while those in the higher dose groups remained on their assigned therapies.
F-VASI Almost Doubled in Extension Trial
From week 24 to week 52, there was nearly a doubling of the percent F-VASI reduction, climbing from 32% to 60.8% in the 11-mg group and from 38.7% to 64.9% in the 22-mg group, Dr. Passeron said. Placebo groups who were switched to active therapy at 24 weeks rapidly approached the rates of F-VASI response of those initiated on upadacitinib.
The percent reductions in T-VASI, although lower, followed the same pattern. For the 11-mg group, the reduction climbed from 16% at 24 weeks to 44.7% at 52 weeks. For the 22-mg group, the reduction climbed from 22.9% to 44.4%. Patients who were switched from placebo to 11 mg or to 22 mg also experienced improvements in T-VASI up to 52 weeks, although the level of improvement was lower than that in patients initially randomized to the higher doses of upadacitinib.
There were “no new safety signals” for upadacitinib, which is FDA-approved for multiple indications, according to Dr. Passeron. He said acne-like lesions were the most bothersome adverse event, and cases of herpes zoster were “rare.”
A version of these data was published in a British Journal of Dermatology supplement just prior to the AAD meeting.
Phase 3 vitiligo trials are planned for both ritlecitinib and upadacitinib.
Dr. Guttman-Yassky reported financial relationships with approximately 45 pharmaceutical companies, including Pfizer, which makes ritlecitinib and provided funding for the study she discussed. Dr. Passeron reported financial relationships with approximately 40 pharmaceutical companies, including AbbVie, which makes upadacitinib and provided funding for the study he discussed.
A version of this article appeared on Medscape.com.
SAN DIEGO — according to presentations at a late-breaking session at the annual meeting of the American Academy of Dermatology (AAD).
In one, the addition of narrow-band ultraviolet-B (NB-UVB) light therapy to ritlecitinib appears more effective than ritlecitinib alone. In the other study, the effectiveness of upadacitinib appears to improve over time.
Based on the ritlecitinib data, “if you have phototherapy in your office, it might be good to couple it with ritlecitinib for vitiligo patients,” said Emma Guttman-Yassky, MD, PhD, chair of the Department of Dermatology, Icahn School of Medicine at Mount Sinai, New York City, who presented the findings.
However, because of the relatively small numbers in the extension study, Dr. Guttman-Yassky characterized the evidence as preliminary and in need of further investigation.
For vitiligo, the only approved JAK inhibitor is ruxolitinib, 1.5%, in a cream formulation. In June, ritlecitinib (Litfulo) was approved by the Food and Drug Administration (FDA) for alopecia areata. Phototherapy, which has been used for decades in the treatment of vitiligo, has an established efficacy and safety profile as a stand-alone vitiligo treatment. Upadacitinib has numerous indications for inflammatory diseases, such as rheumatoid arthritis, and was granted FDA approval for atopic dermatitis in 2022.
NB-UVB Arm Added in Ritlecitinib Extension
The ritlecitinib study population was drawn from patients with non-segmental vitiligo who initially participated in a 24-week dose-ranging period of a phase 2b trial published last year. In that study, 364 patients were randomized to doses of once-daily ritlecitinib ranging from 10 to 50 mg with or without a 4-week loading regimen. Higher doses were generally associated with greater efficacy on the primary endpoint of facial vitiligo area scoring index (F-VASI) but not with a greater risk for adverse events.
In the 24-week extension study, 187 patients received a 4-week loading regimen of 200-mg ritlecitinib daily followed by 50 mg of daily ritlecitinib for the remaining 20 weeks. Another 43 patients were randomized to one of two arms: The same 4-week loading regimen of 200-mg ritlecitinib daily followed by 50 mg of daily ritlecitinib or to 50-mg daily ritlecitinib without a loading dose but combined with NB-UVB delivered twice per week.
Important to interpretation of results, there was an additional twist. Patients in the randomized arm who had < 10% improvement in the total vitiligo area severity index (T-VASI) at week 12 of the extension were discontinued from the study.
The endpoints considered when comparing ritlecitinib with or without NB-UVB at the end of the extension study were F-VASI, T-VASI, patient global impression of change, and adverse events. Responses were assessed on the basis of both observed and last observation carried forward (LOCF).
Of the 43 people, who were randomized in the extension study, nine (21%) had < 10% improvement in T-VASI and were therefore discontinued from the study.
At the end of 24 weeks, both groups had a substantial response to their assigned therapy, but the addition of NB-UVB increased rates of response, although not always at a level of statistical significance, according to Dr. Guttman-Yassky.
For the percent improvement in F-VASI, specifically, the increase did not reach significance on the basis of LOCF (57.9% vs 51.5%; P = .158) but was highly significant on the basis of observed responses (69.6% vs 55.1%; P = .009). For T-VASI, differences for adjunctive NB-UVB over monotherapy did not reach significance for either observed or LOCF responses, but it was significant for observed responses in a patient global impression of change.
Small Numbers Limit Strength of Ritlecitinib, NB-UVB Evidence
However, Dr. Guttman-Yassky said it is important “to pay attention to the sample sizes” when noting the lack of significance.
The combination appeared safe, and there were no side effects associated with the addition of twice-weekly NB-UVB to ritlecitinib.
She acknowledged that the design of this analysis was “complicated” and that the number of randomized patients was small. She suggested the findings support the potential for benefit from the combination of a JAK inhibitor and NB-UVB, both of which have shown efficacy as monotherapy in previous studies. She indicated that a trial of this combination is reasonable while awaiting a more definitive study.
One of the questions that might be posed in a larger study is the timing of NB-UVB, such as whether it is best reserved for those with inadequate early response to a JAK inhibitor or if optimal results are achieved when a JAK inhibitor and NB-UVB are initiated simultaneously.
Upadacitinib Monotherapy Results
One rationale for initiating therapy with the combination of a JAK inhibitor and NB-UVB is the potential for a more rapid response, but extended results from a second phase 2b study with a different oral JAK inhibitor, upadacitinib, suggested responses on JAK inhibitor monotherapy improve steadily over time.
“The overall efficacy continued to improve without reaching a plateau at 1 year,” reported Thierry Passeron, MD, PhD, professor and chair, Department of Dermatology, Université Côte d’Azur, Nice, France. He spoke at the same AAD late-breaking session as Dr. Guttman-Yassky.
The 24-week dose-ranging data from the upadacitinib trial were previously reported at the 2023 annual meeting of the European Association of Dermatology and Venereology. In the placebo-controlled portion, which randomized 185 patients with extensive non-segmental vitiligo to 6 mg, 11 mg, or 22 mg, the two higher doses were significantly more effective than placebo.
In the extension, patients in the placebo group were randomized to 11 mg or 22 mg, while those in the higher dose groups remained on their assigned therapies.
F-VASI Almost Doubled in Extension Trial
From week 24 to week 52, there was nearly a doubling of the percent F-VASI reduction, climbing from 32% to 60.8% in the 11-mg group and from 38.7% to 64.9% in the 22-mg group, Dr. Passeron said. Placebo groups who were switched to active therapy at 24 weeks rapidly approached the rates of F-VASI response of those initiated on upadacitinib.
The percent reductions in T-VASI, although lower, followed the same pattern. For the 11-mg group, the reduction climbed from 16% at 24 weeks to 44.7% at 52 weeks. For the 22-mg group, the reduction climbed from 22.9% to 44.4%. Patients who were switched from placebo to 11 mg or to 22 mg also experienced improvements in T-VASI up to 52 weeks, although the level of improvement was lower than that in patients initially randomized to the higher doses of upadacitinib.
There were “no new safety signals” for upadacitinib, which is FDA-approved for multiple indications, according to Dr. Passeron. He said acne-like lesions were the most bothersome adverse event, and cases of herpes zoster were “rare.”
A version of these data was published in a British Journal of Dermatology supplement just prior to the AAD meeting.
Phase 3 vitiligo trials are planned for both ritlecitinib and upadacitinib.
Dr. Guttman-Yassky reported financial relationships with approximately 45 pharmaceutical companies, including Pfizer, which makes ritlecitinib and provided funding for the study she discussed. Dr. Passeron reported financial relationships with approximately 40 pharmaceutical companies, including AbbVie, which makes upadacitinib and provided funding for the study he discussed.
A version of this article appeared on Medscape.com.
SAN DIEGO — according to presentations at a late-breaking session at the annual meeting of the American Academy of Dermatology (AAD).
In one, the addition of narrow-band ultraviolet-B (NB-UVB) light therapy to ritlecitinib appears more effective than ritlecitinib alone. In the other study, the effectiveness of upadacitinib appears to improve over time.
Based on the ritlecitinib data, “if you have phototherapy in your office, it might be good to couple it with ritlecitinib for vitiligo patients,” said Emma Guttman-Yassky, MD, PhD, chair of the Department of Dermatology, Icahn School of Medicine at Mount Sinai, New York City, who presented the findings.
However, because of the relatively small numbers in the extension study, Dr. Guttman-Yassky characterized the evidence as preliminary and in need of further investigation.
For vitiligo, the only approved JAK inhibitor is ruxolitinib, 1.5%, in a cream formulation. In June, ritlecitinib (Litfulo) was approved by the Food and Drug Administration (FDA) for alopecia areata. Phototherapy, which has been used for decades in the treatment of vitiligo, has an established efficacy and safety profile as a stand-alone vitiligo treatment. Upadacitinib has numerous indications for inflammatory diseases, such as rheumatoid arthritis, and was granted FDA approval for atopic dermatitis in 2022.
NB-UVB Arm Added in Ritlecitinib Extension
The ritlecitinib study population was drawn from patients with non-segmental vitiligo who initially participated in a 24-week dose-ranging period of a phase 2b trial published last year. In that study, 364 patients were randomized to doses of once-daily ritlecitinib ranging from 10 to 50 mg with or without a 4-week loading regimen. Higher doses were generally associated with greater efficacy on the primary endpoint of facial vitiligo area scoring index (F-VASI) but not with a greater risk for adverse events.
In the 24-week extension study, 187 patients received a 4-week loading regimen of 200-mg ritlecitinib daily followed by 50 mg of daily ritlecitinib for the remaining 20 weeks. Another 43 patients were randomized to one of two arms: The same 4-week loading regimen of 200-mg ritlecitinib daily followed by 50 mg of daily ritlecitinib or to 50-mg daily ritlecitinib without a loading dose but combined with NB-UVB delivered twice per week.
Important to interpretation of results, there was an additional twist. Patients in the randomized arm who had < 10% improvement in the total vitiligo area severity index (T-VASI) at week 12 of the extension were discontinued from the study.
The endpoints considered when comparing ritlecitinib with or without NB-UVB at the end of the extension study were F-VASI, T-VASI, patient global impression of change, and adverse events. Responses were assessed on the basis of both observed and last observation carried forward (LOCF).
Of the 43 people, who were randomized in the extension study, nine (21%) had < 10% improvement in T-VASI and were therefore discontinued from the study.
At the end of 24 weeks, both groups had a substantial response to their assigned therapy, but the addition of NB-UVB increased rates of response, although not always at a level of statistical significance, according to Dr. Guttman-Yassky.
For the percent improvement in F-VASI, specifically, the increase did not reach significance on the basis of LOCF (57.9% vs 51.5%; P = .158) but was highly significant on the basis of observed responses (69.6% vs 55.1%; P = .009). For T-VASI, differences for adjunctive NB-UVB over monotherapy did not reach significance for either observed or LOCF responses, but it was significant for observed responses in a patient global impression of change.
Small Numbers Limit Strength of Ritlecitinib, NB-UVB Evidence
However, Dr. Guttman-Yassky said it is important “to pay attention to the sample sizes” when noting the lack of significance.
The combination appeared safe, and there were no side effects associated with the addition of twice-weekly NB-UVB to ritlecitinib.
She acknowledged that the design of this analysis was “complicated” and that the number of randomized patients was small. She suggested the findings support the potential for benefit from the combination of a JAK inhibitor and NB-UVB, both of which have shown efficacy as monotherapy in previous studies. She indicated that a trial of this combination is reasonable while awaiting a more definitive study.
One of the questions that might be posed in a larger study is the timing of NB-UVB, such as whether it is best reserved for those with inadequate early response to a JAK inhibitor or if optimal results are achieved when a JAK inhibitor and NB-UVB are initiated simultaneously.
Upadacitinib Monotherapy Results
One rationale for initiating therapy with the combination of a JAK inhibitor and NB-UVB is the potential for a more rapid response, but extended results from a second phase 2b study with a different oral JAK inhibitor, upadacitinib, suggested responses on JAK inhibitor monotherapy improve steadily over time.
“The overall efficacy continued to improve without reaching a plateau at 1 year,” reported Thierry Passeron, MD, PhD, professor and chair, Department of Dermatology, Université Côte d’Azur, Nice, France. He spoke at the same AAD late-breaking session as Dr. Guttman-Yassky.
The 24-week dose-ranging data from the upadacitinib trial were previously reported at the 2023 annual meeting of the European Association of Dermatology and Venereology. In the placebo-controlled portion, which randomized 185 patients with extensive non-segmental vitiligo to 6 mg, 11 mg, or 22 mg, the two higher doses were significantly more effective than placebo.
In the extension, patients in the placebo group were randomized to 11 mg or 22 mg, while those in the higher dose groups remained on their assigned therapies.
F-VASI Almost Doubled in Extension Trial
From week 24 to week 52, there was nearly a doubling of the percent F-VASI reduction, climbing from 32% to 60.8% in the 11-mg group and from 38.7% to 64.9% in the 22-mg group, Dr. Passeron said. Placebo groups who were switched to active therapy at 24 weeks rapidly approached the rates of F-VASI response of those initiated on upadacitinib.
The percent reductions in T-VASI, although lower, followed the same pattern. For the 11-mg group, the reduction climbed from 16% at 24 weeks to 44.7% at 52 weeks. For the 22-mg group, the reduction climbed from 22.9% to 44.4%. Patients who were switched from placebo to 11 mg or to 22 mg also experienced improvements in T-VASI up to 52 weeks, although the level of improvement was lower than that in patients initially randomized to the higher doses of upadacitinib.
There were “no new safety signals” for upadacitinib, which is FDA-approved for multiple indications, according to Dr. Passeron. He said acne-like lesions were the most bothersome adverse event, and cases of herpes zoster were “rare.”
A version of these data was published in a British Journal of Dermatology supplement just prior to the AAD meeting.
Phase 3 vitiligo trials are planned for both ritlecitinib and upadacitinib.
Dr. Guttman-Yassky reported financial relationships with approximately 45 pharmaceutical companies, including Pfizer, which makes ritlecitinib and provided funding for the study she discussed. Dr. Passeron reported financial relationships with approximately 40 pharmaceutical companies, including AbbVie, which makes upadacitinib and provided funding for the study he discussed.
A version of this article appeared on Medscape.com.
FROM AAD 2024
Trauma, Racism Linked to Increased Suicide Risk in Black Men
One in three Black men in rural America experienced suicidal or death ideation (SDI) in the past week, new research showed.
A developmental model used in the study showed a direct association between experiences pertaining to threat, deprivation, and racial discrimination during childhood and suicide risk in adulthood, suggesting that a broad range of adverse experiences in early life may affect SDI risk among Black men.
“During the past 20-30 years, young Black men have evinced increasing levels of suicidal behavior and related cognitions,” lead author Steven Kogan, PhD, professor of family and consumer sciences at the University of Georgia, Athens, Georgia, and colleagues wrote.
“By controlling for depressive symptoms in assessing increases in SDI over time, our study’s design directly informed the extent to which social adversities affect SDI independent of other depressive problems,” they added.
The findings were published online in Cultural Diversity and Ethnic Minority Psychology.
Second Leading Cause of Death
Suicide is the second leading cause of death for Black Americans ages 15-24, according to the Centers for Disease Control and Prevention. The outlook is worse for Black men, whose death rate from suicide is about four times greater than for Black women.
Previous research suggests Black men are disproportionately exposed to social adversity, including poverty and discrimination, which may increase the risk for SDI. In addition, racial discrimination has been shown to increase the risks for depression, anxiety, and psychological distress among Black youth and adults.
But little research exists to better understand how these negative experiences affect vulnerability to SDI. The new study tested a model linking adversity during childhood and emerging exposure to racial discrimination to increases in suicidal thoughts.
Researchers analyzed data from 504 participants in the African American Men’s Project, which included a series of surveys completed by young men in rural Georgia at three different time points over a period of about 3 years.
Composite scores for childhood threat and deprivation were developed using the Adverse Childhood Experiences Scale and Childhood Trauma Questionnaire. Everyday discrimination was measured on the Schedule of Racist Events response scale.
To assess their experience with childhood threats, the men in the study, who were about 21 years old on average when they enrolled, were asked if they experienced a series of adverse childhood experiences and deprivation through age 16. Questions explored issues such as directly experiencing physical violence or witnessing abuse in the home and whether the men felt loved and “important or special” as children.
The investigators also asked the men about their experiences of racial discrimination, the quality of their relationships, their belief that aggression is a means of gaining respect, and their cynicism regarding romantic relationships.
Targeted Prevention
Overall, 33.6% of participants reported SDI in the previous week. A history of childhood threats and deprivation was associated with an increased likelihood of SDI (P < .001).
Researchers also found that a history of racial discrimination was significantly associated with the development of negative relational schemas, which are characterized by beliefs that other people are untrustworthy, uncaring, and/or hostile. Negative schemas were in turn associated with an increased risk for suicidal thoughts (P = .03).
“Clinical and preventive interventions for suicidality should target the influence of racism and adverse experiences and the negative relational schemas they induce,” the investigators noted.
“Policy efforts designed to dismantle systemic racism are critically needed. Interventions that address SDI, including programming designed to support Black men through their experiences with racial discrimination and processing of childhood experiences of adversity, may help young Black men resist the psychological impacts of racism, expand their positive support networks, and decrease their risk of SDI,” they added.
The study authors reported no funding sources or relevant financial relationships.
A version of this article appeared on Medscape.com.
One in three Black men in rural America experienced suicidal or death ideation (SDI) in the past week, new research showed.
A developmental model used in the study showed a direct association between experiences pertaining to threat, deprivation, and racial discrimination during childhood and suicide risk in adulthood, suggesting that a broad range of adverse experiences in early life may affect SDI risk among Black men.
“During the past 20-30 years, young Black men have evinced increasing levels of suicidal behavior and related cognitions,” lead author Steven Kogan, PhD, professor of family and consumer sciences at the University of Georgia, Athens, Georgia, and colleagues wrote.
“By controlling for depressive symptoms in assessing increases in SDI over time, our study’s design directly informed the extent to which social adversities affect SDI independent of other depressive problems,” they added.
The findings were published online in Cultural Diversity and Ethnic Minority Psychology.
Second Leading Cause of Death
Suicide is the second leading cause of death for Black Americans ages 15-24, according to the Centers for Disease Control and Prevention. The outlook is worse for Black men, whose death rate from suicide is about four times greater than for Black women.
Previous research suggests Black men are disproportionately exposed to social adversity, including poverty and discrimination, which may increase the risk for SDI. In addition, racial discrimination has been shown to increase the risks for depression, anxiety, and psychological distress among Black youth and adults.
But little research exists to better understand how these negative experiences affect vulnerability to SDI. The new study tested a model linking adversity during childhood and emerging exposure to racial discrimination to increases in suicidal thoughts.
Researchers analyzed data from 504 participants in the African American Men’s Project, which included a series of surveys completed by young men in rural Georgia at three different time points over a period of about 3 years.
Composite scores for childhood threat and deprivation were developed using the Adverse Childhood Experiences Scale and Childhood Trauma Questionnaire. Everyday discrimination was measured on the Schedule of Racist Events response scale.
To assess their experience with childhood threats, the men in the study, who were about 21 years old on average when they enrolled, were asked if they experienced a series of adverse childhood experiences and deprivation through age 16. Questions explored issues such as directly experiencing physical violence or witnessing abuse in the home and whether the men felt loved and “important or special” as children.
The investigators also asked the men about their experiences of racial discrimination, the quality of their relationships, their belief that aggression is a means of gaining respect, and their cynicism regarding romantic relationships.
Targeted Prevention
Overall, 33.6% of participants reported SDI in the previous week. A history of childhood threats and deprivation was associated with an increased likelihood of SDI (P < .001).
Researchers also found that a history of racial discrimination was significantly associated with the development of negative relational schemas, which are characterized by beliefs that other people are untrustworthy, uncaring, and/or hostile. Negative schemas were in turn associated with an increased risk for suicidal thoughts (P = .03).
“Clinical and preventive interventions for suicidality should target the influence of racism and adverse experiences and the negative relational schemas they induce,” the investigators noted.
“Policy efforts designed to dismantle systemic racism are critically needed. Interventions that address SDI, including programming designed to support Black men through their experiences with racial discrimination and processing of childhood experiences of adversity, may help young Black men resist the psychological impacts of racism, expand their positive support networks, and decrease their risk of SDI,” they added.
The study authors reported no funding sources or relevant financial relationships.
A version of this article appeared on Medscape.com.
One in three Black men in rural America experienced suicidal or death ideation (SDI) in the past week, new research showed.
A developmental model used in the study showed a direct association between experiences pertaining to threat, deprivation, and racial discrimination during childhood and suicide risk in adulthood, suggesting that a broad range of adverse experiences in early life may affect SDI risk among Black men.
“During the past 20-30 years, young Black men have evinced increasing levels of suicidal behavior and related cognitions,” lead author Steven Kogan, PhD, professor of family and consumer sciences at the University of Georgia, Athens, Georgia, and colleagues wrote.
“By controlling for depressive symptoms in assessing increases in SDI over time, our study’s design directly informed the extent to which social adversities affect SDI independent of other depressive problems,” they added.
The findings were published online in Cultural Diversity and Ethnic Minority Psychology.
Second Leading Cause of Death
Suicide is the second leading cause of death for Black Americans ages 15-24, according to the Centers for Disease Control and Prevention. The outlook is worse for Black men, whose death rate from suicide is about four times greater than for Black women.
Previous research suggests Black men are disproportionately exposed to social adversity, including poverty and discrimination, which may increase the risk for SDI. In addition, racial discrimination has been shown to increase the risks for depression, anxiety, and psychological distress among Black youth and adults.
But little research exists to better understand how these negative experiences affect vulnerability to SDI. The new study tested a model linking adversity during childhood and emerging exposure to racial discrimination to increases in suicidal thoughts.
Researchers analyzed data from 504 participants in the African American Men’s Project, which included a series of surveys completed by young men in rural Georgia at three different time points over a period of about 3 years.
Composite scores for childhood threat and deprivation were developed using the Adverse Childhood Experiences Scale and Childhood Trauma Questionnaire. Everyday discrimination was measured on the Schedule of Racist Events response scale.
To assess their experience with childhood threats, the men in the study, who were about 21 years old on average when they enrolled, were asked if they experienced a series of adverse childhood experiences and deprivation through age 16. Questions explored issues such as directly experiencing physical violence or witnessing abuse in the home and whether the men felt loved and “important or special” as children.
The investigators also asked the men about their experiences of racial discrimination, the quality of their relationships, their belief that aggression is a means of gaining respect, and their cynicism regarding romantic relationships.
Targeted Prevention
Overall, 33.6% of participants reported SDI in the previous week. A history of childhood threats and deprivation was associated with an increased likelihood of SDI (P < .001).
Researchers also found that a history of racial discrimination was significantly associated with the development of negative relational schemas, which are characterized by beliefs that other people are untrustworthy, uncaring, and/or hostile. Negative schemas were in turn associated with an increased risk for suicidal thoughts (P = .03).
“Clinical and preventive interventions for suicidality should target the influence of racism and adverse experiences and the negative relational schemas they induce,” the investigators noted.
“Policy efforts designed to dismantle systemic racism are critically needed. Interventions that address SDI, including programming designed to support Black men through their experiences with racial discrimination and processing of childhood experiences of adversity, may help young Black men resist the psychological impacts of racism, expand their positive support networks, and decrease their risk of SDI,” they added.
The study authors reported no funding sources or relevant financial relationships.
A version of this article appeared on Medscape.com.
FROM CULTURAL DIVERSITY AND ETHNIC MINORITY PSYCHOLOGY
Do Tumor-infiltrating Lymphocytes Predict Better Breast Cancer Outcomes?
The association of abundant tumor-infiltrating lymphocytes (TILs) in breast cancer tissue with outcomes in patients with early-stage triple-negative breast cancer (TNBC) who do not receive chemotherapy has not been well studied, wrote Roberto A. Leon-Ferre, MD, of the Mayo Clinic, Rochester, Minnesota, and colleagues, in JAMA.
Biomarkers to guide systemic treatment and avoid overtreatment are lacking, and such markers could help identify patients who could achieve increased survival with less intensive therapy, continued the authors of the new study of nearly 2000 individuals.
“TNBC is the most aggressive subtype of breast cancer, and for this reason, current treatment guidelines recommend chemotherapy using multiple drugs either before or after surgery,” Dr. Leon-Ferre said in an interview. “We have learned over the last several years that TNBC is not a single disease, but that there are several subtypes of TNBC that have different risks and different vulnerabilities, and treating all patients similarly may not be optimal.”
What is Known About Tumor-Infiltrating Lymphocytes and Cancer?
Previous studies have shown improved survival in patients with early-stage TNBC and high levels of TILs who were treated with adjuvant and neoadjuvant chemotherapy, compared with those with lower TILs. In a pooled analysis of 2148 patients from nine studies published in the Journal of Clinical Oncology in 2019, a higher percentage of TILs in the stroma surrounding a tumor was significantly associated with improved survival in TNBC patients after adjuvant chemotherapy.
Another study published in the Journal of Clinical Oncology in 2022 showed that elevated TILs were significant predictors of overall survival, but the study included fewer than 500 patients.
The potential mechanisms that drive the association between elevated TILs and improved survival include the ability of TILs to attack cancer cells, Dr. Leon-Ferre said in an interview.
The goal of this study was to evaluate whether TILs could identify a subset of patients with TNBC who had a very low risk of cancer recurrence even if chemotherapy was not given.
“Indeed, we found that patients with stage I TNBC and high TILs had a very low risk of recurrence even when chemotherapy was not administered. These findings will pave the way for future studies aiming to reduce the need for multiple chemotherapy drugs in patients with stage I TNBC and decrease the side effects that patients face,” he said.
What Does the New Study Add?
The current study included 1966 individuals from 13 sites in North America, Europe, and Asia who were diagnosed with TNBC between 1979 and 2017 and were treated with surgery, with or without radiotherapy but with no adjuvant or neoadjuvant chemotherapy. The researchers examined the abundance of TILs in the breast tissue of resected primary tumors; the primary outcome was invasive disease-free survival (iDFS), with recurrence-free survival, distant recurrence-free survival, and overall survival as secondary outcomes.
The median age of the patients was 56 years, 55% had stage I TNBC, and the median TIL level was 15%.
A total of 417 patients had a TIL level of 50% or more, and the 5-year iDFS for these patients was 94%, compared with 78% for those with a TIL level less than 30%. Similarly, 5-year overall survival was 95% in patients with a TIL level of 50% or more, compared with 82% for patients with TIL levels of less than 30%.
Additionally, each 10% increase in TILs was independently associated not only with improved iDFS (hazard ratio[HR], 0.92), but also improved recurrence-free survival (HR, 0.90), distant recurrence-free survival (HR, 0.87), and overall survival (HR, 0.88) over a median follow-up of 18 years.
The current study shows that cancer stage based on tumor size and the number of lymph nodes should not be the only considerations for making treatment decisions and predicting outcomes, Dr. Leon-Ferre said in an interview.
“In fact, our study shows that for tumors of the same stage (particularly for stage I), the risk of recurrence is different depending on the number of TILs seen in the breast cancer tissue. When chemo is not given, those with high TILs have lower risk of recurrence, whereas those with low TILs have a higher risk of recurrence,” he said.
What are the Limitations of This Research?
The current study findings are limited by the retrospective design and use of observational data, so the researchers could not make conclusions about causality. Other limitations included lack of data on germline mutations and race or ethnicity, and the potential irrelevance of data from patients treated as long as 45 years ago.
“Because most patients with TNBC receive chemotherapy in the modern times, we needed to work with 13 hospitals around the world to find data on patients with TNBC who never received chemotherapy for various reasons,” Dr. Leon-Ferre said.
To address these limitations, Dr. Leone-Ferre and his colleagues are planning prospective studies where TILs will be used to make treatment decisions.
“Many of the patients in our cohort were treated many years ago, when chemotherapy was not routinely given. Advances in cancer detection, surgical and radiation techniques may lead to different results in patients treated today,” he added.
What Do Oncologists Need to Know?
The current study findings may provide additional information on prognosis that is important to share with patients for decision-making on the risks versus benefits of chemotherapy, Dr. Leon-Ferre said.
“Like any test, TILs should not be used in isolation to make decisions, but should be integrated with other factors including the cancer stage, the overall patient health, patient preferences, and concerns about treatment complications,” he emphasized. “The results of this study allow oncologists to offer a more refined calculation of recurrence risk if patients opt to not receive chemotherapy.”
In the current study, although younger age was associated with higher TIL levels, a finding consistent with previous studies, increased TIL, remained significantly associated with improved survival after adjusting for age, tumor size, nodal status, and histological grade.
Overall, “the findings suggest that for patients with stage I TNBC and TILs greater than 50%, chemotherapy may not be as necessary as it was previously thought,” Dr. Leon-Ferre said.
What Additional Research is Needed?
Prospective studies are needed to validate the findings, including studies in diverse populations, and additional studies may investigate whether early TBNC patients with high TIL levels could achieve high cure rates with less intensive and less toxic chemotherapy regiments than those currently recommended, the researchers wrote in their discussion.
“There are many additional research questions that we need to answer, and look forward to working on,” Dr. Leon-Ferre said, in an interview. These topics include whether TILs can be used to decide on the number of chemotherapy drugs a patient really needs and whether artificial intelligence can be used to evaluate TILs more quickly and effectively than the human eye, he said. Other research topics include identifying which particular type of TILs attack cancer cells most effectively and whether TILs could be increased in patients with low levels in order to improve their prognosis, he added.
The study was supported by the National Research Agency and General Secretariat for Investment, Clinical and Translational Science Awards, the Mayo Clinic Breast Cancer SPORE grant, the Cancer Research Society of Canada, institutional grants from the Dutch Cancer Society, The Netherlands Organization for Health Research, and several foundations. Dr. Leon-Ferre disclosed consulting honoraria to his institution for research activities from AstraZeneca, Gilead Sciences, and Lyell Immunopharma, with no personal fees outside the submitted work.
The association of abundant tumor-infiltrating lymphocytes (TILs) in breast cancer tissue with outcomes in patients with early-stage triple-negative breast cancer (TNBC) who do not receive chemotherapy has not been well studied, wrote Roberto A. Leon-Ferre, MD, of the Mayo Clinic, Rochester, Minnesota, and colleagues, in JAMA.
Biomarkers to guide systemic treatment and avoid overtreatment are lacking, and such markers could help identify patients who could achieve increased survival with less intensive therapy, continued the authors of the new study of nearly 2000 individuals.
“TNBC is the most aggressive subtype of breast cancer, and for this reason, current treatment guidelines recommend chemotherapy using multiple drugs either before or after surgery,” Dr. Leon-Ferre said in an interview. “We have learned over the last several years that TNBC is not a single disease, but that there are several subtypes of TNBC that have different risks and different vulnerabilities, and treating all patients similarly may not be optimal.”
What is Known About Tumor-Infiltrating Lymphocytes and Cancer?
Previous studies have shown improved survival in patients with early-stage TNBC and high levels of TILs who were treated with adjuvant and neoadjuvant chemotherapy, compared with those with lower TILs. In a pooled analysis of 2148 patients from nine studies published in the Journal of Clinical Oncology in 2019, a higher percentage of TILs in the stroma surrounding a tumor was significantly associated with improved survival in TNBC patients after adjuvant chemotherapy.
Another study published in the Journal of Clinical Oncology in 2022 showed that elevated TILs were significant predictors of overall survival, but the study included fewer than 500 patients.
The potential mechanisms that drive the association between elevated TILs and improved survival include the ability of TILs to attack cancer cells, Dr. Leon-Ferre said in an interview.
The goal of this study was to evaluate whether TILs could identify a subset of patients with TNBC who had a very low risk of cancer recurrence even if chemotherapy was not given.
“Indeed, we found that patients with stage I TNBC and high TILs had a very low risk of recurrence even when chemotherapy was not administered. These findings will pave the way for future studies aiming to reduce the need for multiple chemotherapy drugs in patients with stage I TNBC and decrease the side effects that patients face,” he said.
What Does the New Study Add?
The current study included 1966 individuals from 13 sites in North America, Europe, and Asia who were diagnosed with TNBC between 1979 and 2017 and were treated with surgery, with or without radiotherapy but with no adjuvant or neoadjuvant chemotherapy. The researchers examined the abundance of TILs in the breast tissue of resected primary tumors; the primary outcome was invasive disease-free survival (iDFS), with recurrence-free survival, distant recurrence-free survival, and overall survival as secondary outcomes.
The median age of the patients was 56 years, 55% had stage I TNBC, and the median TIL level was 15%.
A total of 417 patients had a TIL level of 50% or more, and the 5-year iDFS for these patients was 94%, compared with 78% for those with a TIL level less than 30%. Similarly, 5-year overall survival was 95% in patients with a TIL level of 50% or more, compared with 82% for patients with TIL levels of less than 30%.
Additionally, each 10% increase in TILs was independently associated not only with improved iDFS (hazard ratio[HR], 0.92), but also improved recurrence-free survival (HR, 0.90), distant recurrence-free survival (HR, 0.87), and overall survival (HR, 0.88) over a median follow-up of 18 years.
The current study shows that cancer stage based on tumor size and the number of lymph nodes should not be the only considerations for making treatment decisions and predicting outcomes, Dr. Leon-Ferre said in an interview.
“In fact, our study shows that for tumors of the same stage (particularly for stage I), the risk of recurrence is different depending on the number of TILs seen in the breast cancer tissue. When chemo is not given, those with high TILs have lower risk of recurrence, whereas those with low TILs have a higher risk of recurrence,” he said.
What are the Limitations of This Research?
The current study findings are limited by the retrospective design and use of observational data, so the researchers could not make conclusions about causality. Other limitations included lack of data on germline mutations and race or ethnicity, and the potential irrelevance of data from patients treated as long as 45 years ago.
“Because most patients with TNBC receive chemotherapy in the modern times, we needed to work with 13 hospitals around the world to find data on patients with TNBC who never received chemotherapy for various reasons,” Dr. Leon-Ferre said.
To address these limitations, Dr. Leone-Ferre and his colleagues are planning prospective studies where TILs will be used to make treatment decisions.
“Many of the patients in our cohort were treated many years ago, when chemotherapy was not routinely given. Advances in cancer detection, surgical and radiation techniques may lead to different results in patients treated today,” he added.
What Do Oncologists Need to Know?
The current study findings may provide additional information on prognosis that is important to share with patients for decision-making on the risks versus benefits of chemotherapy, Dr. Leon-Ferre said.
“Like any test, TILs should not be used in isolation to make decisions, but should be integrated with other factors including the cancer stage, the overall patient health, patient preferences, and concerns about treatment complications,” he emphasized. “The results of this study allow oncologists to offer a more refined calculation of recurrence risk if patients opt to not receive chemotherapy.”
In the current study, although younger age was associated with higher TIL levels, a finding consistent with previous studies, increased TIL, remained significantly associated with improved survival after adjusting for age, tumor size, nodal status, and histological grade.
Overall, “the findings suggest that for patients with stage I TNBC and TILs greater than 50%, chemotherapy may not be as necessary as it was previously thought,” Dr. Leon-Ferre said.
What Additional Research is Needed?
Prospective studies are needed to validate the findings, including studies in diverse populations, and additional studies may investigate whether early TBNC patients with high TIL levels could achieve high cure rates with less intensive and less toxic chemotherapy regiments than those currently recommended, the researchers wrote in their discussion.
“There are many additional research questions that we need to answer, and look forward to working on,” Dr. Leon-Ferre said, in an interview. These topics include whether TILs can be used to decide on the number of chemotherapy drugs a patient really needs and whether artificial intelligence can be used to evaluate TILs more quickly and effectively than the human eye, he said. Other research topics include identifying which particular type of TILs attack cancer cells most effectively and whether TILs could be increased in patients with low levels in order to improve their prognosis, he added.
The study was supported by the National Research Agency and General Secretariat for Investment, Clinical and Translational Science Awards, the Mayo Clinic Breast Cancer SPORE grant, the Cancer Research Society of Canada, institutional grants from the Dutch Cancer Society, The Netherlands Organization for Health Research, and several foundations. Dr. Leon-Ferre disclosed consulting honoraria to his institution for research activities from AstraZeneca, Gilead Sciences, and Lyell Immunopharma, with no personal fees outside the submitted work.
The association of abundant tumor-infiltrating lymphocytes (TILs) in breast cancer tissue with outcomes in patients with early-stage triple-negative breast cancer (TNBC) who do not receive chemotherapy has not been well studied, wrote Roberto A. Leon-Ferre, MD, of the Mayo Clinic, Rochester, Minnesota, and colleagues, in JAMA.
Biomarkers to guide systemic treatment and avoid overtreatment are lacking, and such markers could help identify patients who could achieve increased survival with less intensive therapy, continued the authors of the new study of nearly 2000 individuals.
“TNBC is the most aggressive subtype of breast cancer, and for this reason, current treatment guidelines recommend chemotherapy using multiple drugs either before or after surgery,” Dr. Leon-Ferre said in an interview. “We have learned over the last several years that TNBC is not a single disease, but that there are several subtypes of TNBC that have different risks and different vulnerabilities, and treating all patients similarly may not be optimal.”
What is Known About Tumor-Infiltrating Lymphocytes and Cancer?
Previous studies have shown improved survival in patients with early-stage TNBC and high levels of TILs who were treated with adjuvant and neoadjuvant chemotherapy, compared with those with lower TILs. In a pooled analysis of 2148 patients from nine studies published in the Journal of Clinical Oncology in 2019, a higher percentage of TILs in the stroma surrounding a tumor was significantly associated with improved survival in TNBC patients after adjuvant chemotherapy.
Another study published in the Journal of Clinical Oncology in 2022 showed that elevated TILs were significant predictors of overall survival, but the study included fewer than 500 patients.
The potential mechanisms that drive the association between elevated TILs and improved survival include the ability of TILs to attack cancer cells, Dr. Leon-Ferre said in an interview.
The goal of this study was to evaluate whether TILs could identify a subset of patients with TNBC who had a very low risk of cancer recurrence even if chemotherapy was not given.
“Indeed, we found that patients with stage I TNBC and high TILs had a very low risk of recurrence even when chemotherapy was not administered. These findings will pave the way for future studies aiming to reduce the need for multiple chemotherapy drugs in patients with stage I TNBC and decrease the side effects that patients face,” he said.
What Does the New Study Add?
The current study included 1966 individuals from 13 sites in North America, Europe, and Asia who were diagnosed with TNBC between 1979 and 2017 and were treated with surgery, with or without radiotherapy but with no adjuvant or neoadjuvant chemotherapy. The researchers examined the abundance of TILs in the breast tissue of resected primary tumors; the primary outcome was invasive disease-free survival (iDFS), with recurrence-free survival, distant recurrence-free survival, and overall survival as secondary outcomes.
The median age of the patients was 56 years, 55% had stage I TNBC, and the median TIL level was 15%.
A total of 417 patients had a TIL level of 50% or more, and the 5-year iDFS for these patients was 94%, compared with 78% for those with a TIL level less than 30%. Similarly, 5-year overall survival was 95% in patients with a TIL level of 50% or more, compared with 82% for patients with TIL levels of less than 30%.
Additionally, each 10% increase in TILs was independently associated not only with improved iDFS (hazard ratio[HR], 0.92), but also improved recurrence-free survival (HR, 0.90), distant recurrence-free survival (HR, 0.87), and overall survival (HR, 0.88) over a median follow-up of 18 years.
The current study shows that cancer stage based on tumor size and the number of lymph nodes should not be the only considerations for making treatment decisions and predicting outcomes, Dr. Leon-Ferre said in an interview.
“In fact, our study shows that for tumors of the same stage (particularly for stage I), the risk of recurrence is different depending on the number of TILs seen in the breast cancer tissue. When chemo is not given, those with high TILs have lower risk of recurrence, whereas those with low TILs have a higher risk of recurrence,” he said.
What are the Limitations of This Research?
The current study findings are limited by the retrospective design and use of observational data, so the researchers could not make conclusions about causality. Other limitations included lack of data on germline mutations and race or ethnicity, and the potential irrelevance of data from patients treated as long as 45 years ago.
“Because most patients with TNBC receive chemotherapy in the modern times, we needed to work with 13 hospitals around the world to find data on patients with TNBC who never received chemotherapy for various reasons,” Dr. Leon-Ferre said.
To address these limitations, Dr. Leone-Ferre and his colleagues are planning prospective studies where TILs will be used to make treatment decisions.
“Many of the patients in our cohort were treated many years ago, when chemotherapy was not routinely given. Advances in cancer detection, surgical and radiation techniques may lead to different results in patients treated today,” he added.
What Do Oncologists Need to Know?
The current study findings may provide additional information on prognosis that is important to share with patients for decision-making on the risks versus benefits of chemotherapy, Dr. Leon-Ferre said.
“Like any test, TILs should not be used in isolation to make decisions, but should be integrated with other factors including the cancer stage, the overall patient health, patient preferences, and concerns about treatment complications,” he emphasized. “The results of this study allow oncologists to offer a more refined calculation of recurrence risk if patients opt to not receive chemotherapy.”
In the current study, although younger age was associated with higher TIL levels, a finding consistent with previous studies, increased TIL, remained significantly associated with improved survival after adjusting for age, tumor size, nodal status, and histological grade.
Overall, “the findings suggest that for patients with stage I TNBC and TILs greater than 50%, chemotherapy may not be as necessary as it was previously thought,” Dr. Leon-Ferre said.
What Additional Research is Needed?
Prospective studies are needed to validate the findings, including studies in diverse populations, and additional studies may investigate whether early TBNC patients with high TIL levels could achieve high cure rates with less intensive and less toxic chemotherapy regiments than those currently recommended, the researchers wrote in their discussion.
“There are many additional research questions that we need to answer, and look forward to working on,” Dr. Leon-Ferre said, in an interview. These topics include whether TILs can be used to decide on the number of chemotherapy drugs a patient really needs and whether artificial intelligence can be used to evaluate TILs more quickly and effectively than the human eye, he said. Other research topics include identifying which particular type of TILs attack cancer cells most effectively and whether TILs could be increased in patients with low levels in order to improve their prognosis, he added.
The study was supported by the National Research Agency and General Secretariat for Investment, Clinical and Translational Science Awards, the Mayo Clinic Breast Cancer SPORE grant, the Cancer Research Society of Canada, institutional grants from the Dutch Cancer Society, The Netherlands Organization for Health Research, and several foundations. Dr. Leon-Ferre disclosed consulting honoraria to his institution for research activities from AstraZeneca, Gilead Sciences, and Lyell Immunopharma, with no personal fees outside the submitted work.
FROM JAMA
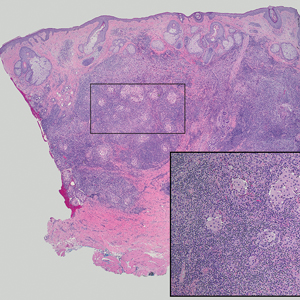
Tender Dermal Nodule on the Temple
The Diagnosis: Lymphoepithelioma-like Carcinoma
Lymphoepithelioma-like carcinoma (LELC) is a rare, poorly differentiated, primary cutaneous neoplasm that occurs on sun-exposed skin, particularly on the head and neck of elderly individuals. It often manifests as an asymptomatic, slow-growing, flesh-colored or erythematous dermal nodule, though ulceration and tenderness have been reported.1 Histopathologically, these neoplasms often are poorly circumscribed and can infiltrate surrounding subcutaneous and soft tissue. As a biphasic tumor, LELC is characterized by islands, nests, or trabeculae of epithelioid cells within the mid dermis surrounded by a dense lymphocytic infiltrate with plasma cells (Figure 1).1 The epithelial component rarely communicates with the overlying epidermis and is composed of atypical polygonal cells with eosinophilic cytoplasm, vesicular nuclei, prominent nucleoli, and frequent mitosis.2 These epithelial nests can be highlighted by pancytokeratin AE1/AE3 or other epithelial differentiation markers (eg, CAM 5.2, CK5/6, epithelial membrane antigen, high-molecular-weight cytokeratin), while the surrounding lymphocytic infiltrate consists of an admixture of T cells and B cells. Lymphoepithelioma-like carcinomas also can demonstrate sebaceous, eccrine, or follicular differentiations.3 The epithelial nests of LELC also are positive for p63 and epithelial membrane antigen.2
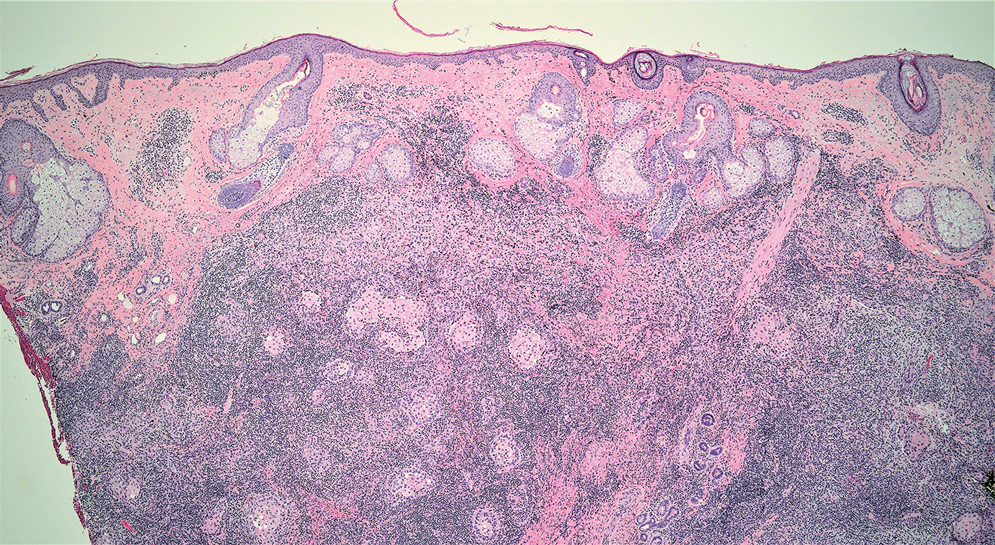
The usual treatment of LELC is wide local excision or Mohs micrographic surgery.1 Despite the poorly differentiated morphology of the tumor, LELC has a generally good prognosis with low metastatic potential and few reports of local recurrence after incomplete excision.3 Patients who are not candidates for surgery as well as recalcitrant cases are managed with radiotherapy.1
Cutaneous lymphadenoma (CL) is a benign adnexal neoplasm that manifests as a small, solitary, fleshcolored nodule usually in the head and neck region.4 Histologically, CL consists of well-circumscribed epithelial nests within the dermis that are peripherally outlined by palisading basaloid cells and filled with clear to eosinophilic epithelioid cells (Figure 2).5 The fibrotic tumor stroma often is infiltrated by numerous intralobular dendritic cells and lymphocytes that occasionally can be arranged in germinal center–like nodules.4 The lymphoepithelial nature of CL can be challenging to distinguish morphologically from LELC, and immunohistochemistry stains may be required. In CL, both the basaloid and epithelioid cells stain positive for pancytokeratin AE1/ AE3, but the peripheral palisaded basaloid cells also stain positive for BerEP4. Additionally, the fibrotic stroma can be highlighted by CD34 and the intralobular dendritic cells by S-100.4
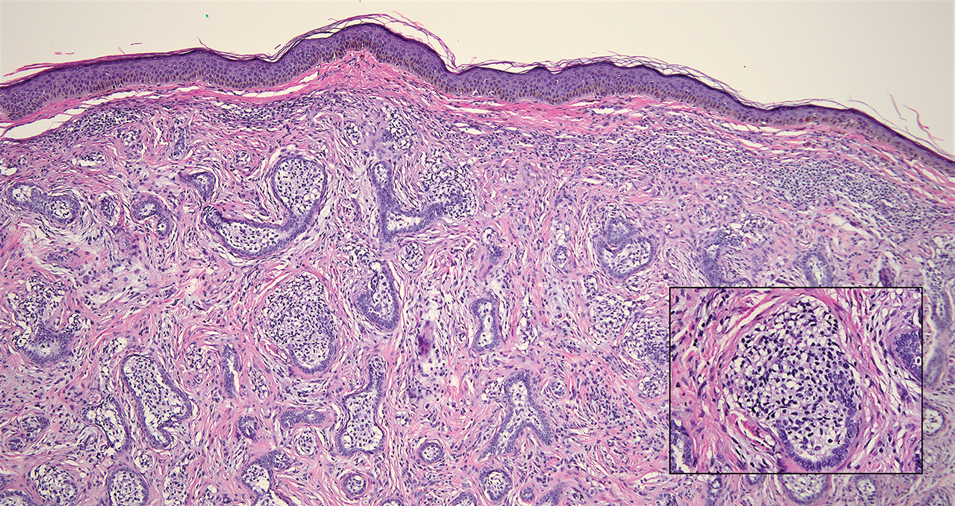
Nasopharyngeal carcinoma (NPC), formerly known as lymphoepithelioma, refers to carcinoma arising within the epithelium of the nasopharynx.6 Endemic to China, NPC manifests as an enlarging nasopharyngeal mass, causing clinical symptoms such as nasal obstruction and epistaxis.7 Histologically, nonkeratinizing NPC exhibits a biphasic morphology consisting of epithelioid neoplastic cells and background lymphocytic infiltrates (Figure 3). The epithelial component consists of round to oval neoplastic cells with amphophilic to eosinophilic cytoplasm, vesicular nuclei, and prominent nucleoli.6 Nasopharyngeal carcinoma is associated strongly with the Epstein-Barr virus while LELC is not; thus, Epstein- Barr encoding region in situ hybridization can reliably distinguish these entities. Metastatic NPC is rare but has been reported; therefore, it is highly recommended to perform an otolaryngologic examination in addition to testing for Epstein-Barr virus reactivity as part of a complete evaluation.8
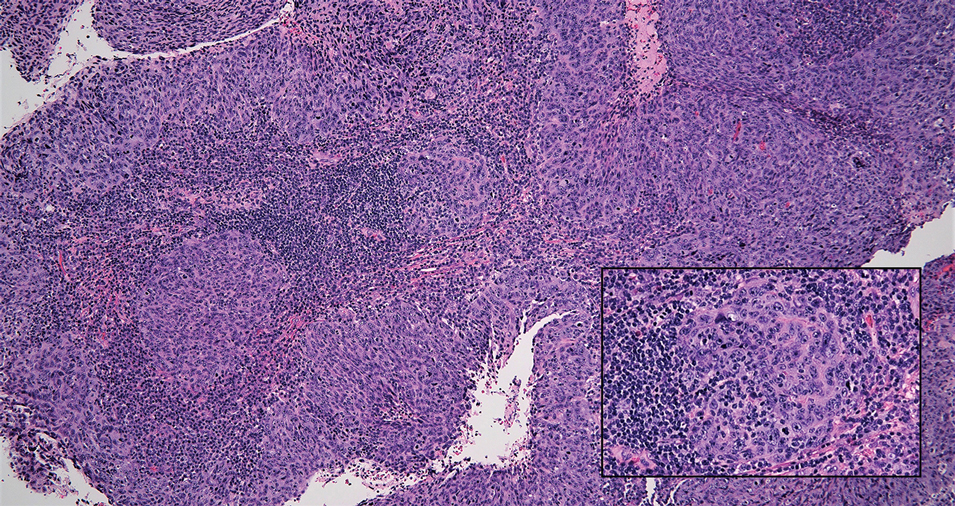
Cutaneous squamous cell carcinoma (SCC) is a common epidermal malignancy with multiple subtypes and variable morphology. The clinical presentation of SCC is similar to LELC—an enlarging hyperkeratotic papule or nodule on sun-exposed skin that often is ulcerated and tender.9 Histologically, poorly differentiated nonkeratinizing SCC can form nests and trabeculae of epithelioid cells that are stained by epithelial differentiation markers, resembling the epithelioid nests of LELC. Distinguishing between LELC and poorly differentiated SCC with robust inflammatory infiltrate can be challenging (Figure 4). In fact, some experts support LELC as an SCC variant rather than a separate entity.9 However, in contrast to LELC, the dermal nests of SCC usually maintain an epidermal connection and often are associated with an overlying area of SCC in situ or welldifferentiated SCC.3
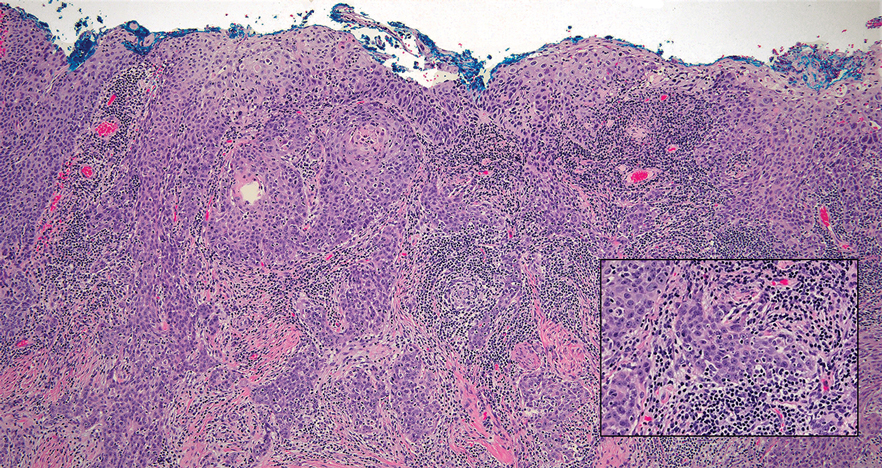
Mycosis fungoides (MF) is a primary cutaneous T-cell lymphoma. It is the most common type of cutaneous lymphoma, accounting for almost 50% of all reported cases.10 Classic MF has an indolent course and progresses through several clinical stages. Patches and plaques characterize early stages; lymphadenopathy indicates progression to later stages in which erythroderma may develop with coalescence of patches, plaques, and tumors; and MF present in blood or lymph nodes characterizes the late stage. Each stage of MF is different histologically—from a superficial lichenoid infiltrate with exocytosis of malignant T cells in the patch stage, to more robust epidermotropism and dermal infiltrate in the plaque stage, and finally a dense dermal infiltrate in the late stage.11 The rare syringotropic variant of MF clinically manifests as solitary or multiple erythematous lesions, often with overlying alopecia. Syringotropic MF uniquely exhibits folliculotropism and syringotropism along with syringometaplasia on histologic evaluation (Figure 5).12 The syringometaplasia can be difficult to distinguish from the epithelial nests of LELC, particularly with the lymphocytic background. Immunohistochemical panels for T-cell markers can highlight aberrant T cells in syringotropic MF through their usual loss of CD5 and CD7, in comparison to normal T cells in LELC.11 An elevated CD4:CD8 ratio of 4:1 and molecular analysis for T-cell receptor gene clonal rearrangements also can support the diagnosis of MF.12
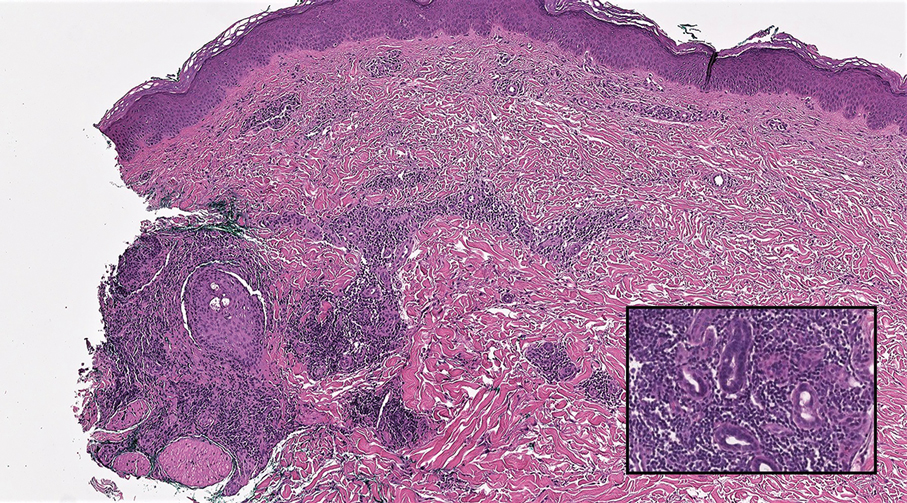
- Morteza Abedi S, Salama S, Alowami S. Lymphoepithelioma-like carcinoma of the skin: case report and approach to surgical pathology sign out. Rare Tumors. 2013;5:E47.
- Fisher JC, White RM, Hurd DS. Lymphoepithelioma-like carcinoma of the skin: a case of one patient presenting with two primary cutaneous neoplasms. J Am Osteopath Coll Dermatol. 2015;33:40-41.
- Welch PQ, Williams SB, Foss RD, et al. Lymphoepithelioma-like carcinoma of head and neck skin: a systematic analysis of 11 cases and review of literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011;111:78-86.
- Yu R, Salama S, Alowami S. Cutaneous lymphadenoma: a rare case and brief review of a diagnostic pitfall. Rare Tumors. 2014;6:5358.
- Monteagudo C, Fúnez R, Sánchez-Sendra B, et al. Cutaneous lymphadenoma is a distinct trichoblastoma-like lymphoepithelial tumor with diffuse androgen receptor immunoreactivity, Notch1 ligand in Reed-Sternberg-like Cells, and common EGFR somatic mutations. Am J Surg Pathol. 2021;45:1382-1390.
- Stelow EB, Wenig BM. Update from the 4th edition of the World Health Organization classification of head and neck tumours: nasopharynx. Head Neck Pathol. 2017;11:16-22.
- Almomani MH, Zulfiqar H, Nagalli S. Nasopharyngeal carcinoma (NPC, lymphoepithelioma). StatPearls Publishing; 2022.
- Lassen CB, Lock-Andersen J. Lymphoepithelioma-like carcinoma of the skin: a case with perineural invasion. Plast Reconstr Surg Glob Open. 2014;2:E252.
- Motaparthi K, Kapil JP, Velazquez EF. Cutaneous squamous cell carcinoma: review of the eighth edition of the American Joint Committee on Cancer Staging Guidelines, Prognostic Factors, and Histopathologic Variants. Adv Anat Pathol. 2017;24:171-194.
- Pileri A, Facchetti F, Rütten A, et al. Syringotropic mycosis fungoides: a rare variant of the disease with peculiar clinicopathologic features. Am J Surg Pathol. 2011;35:100-109.
- Ryu HJ, Kim SI, Jang HO, et al. Evaluation of the International Society for Cutaneous Lymphoma Algorithm for the Diagnosis of Early Mycosis Fungoides [published October 15, 2021]. Cells. 2021;10:2758. doi:10.3390/cells10102758
- Lehmer LM, Amber KT, de Feraudy SM. Syringotropic mycosis fungoides: a rare form of cutaneous T-cell lymphoma enabling a histopathologic “sigh of relief.” Am J Dermatopathol. 2017;39:920-923.
The Diagnosis: Lymphoepithelioma-like Carcinoma
Lymphoepithelioma-like carcinoma (LELC) is a rare, poorly differentiated, primary cutaneous neoplasm that occurs on sun-exposed skin, particularly on the head and neck of elderly individuals. It often manifests as an asymptomatic, slow-growing, flesh-colored or erythematous dermal nodule, though ulceration and tenderness have been reported.1 Histopathologically, these neoplasms often are poorly circumscribed and can infiltrate surrounding subcutaneous and soft tissue. As a biphasic tumor, LELC is characterized by islands, nests, or trabeculae of epithelioid cells within the mid dermis surrounded by a dense lymphocytic infiltrate with plasma cells (Figure 1).1 The epithelial component rarely communicates with the overlying epidermis and is composed of atypical polygonal cells with eosinophilic cytoplasm, vesicular nuclei, prominent nucleoli, and frequent mitosis.2 These epithelial nests can be highlighted by pancytokeratin AE1/AE3 or other epithelial differentiation markers (eg, CAM 5.2, CK5/6, epithelial membrane antigen, high-molecular-weight cytokeratin), while the surrounding lymphocytic infiltrate consists of an admixture of T cells and B cells. Lymphoepithelioma-like carcinomas also can demonstrate sebaceous, eccrine, or follicular differentiations.3 The epithelial nests of LELC also are positive for p63 and epithelial membrane antigen.2

The usual treatment of LELC is wide local excision or Mohs micrographic surgery.1 Despite the poorly differentiated morphology of the tumor, LELC has a generally good prognosis with low metastatic potential and few reports of local recurrence after incomplete excision.3 Patients who are not candidates for surgery as well as recalcitrant cases are managed with radiotherapy.1
Cutaneous lymphadenoma (CL) is a benign adnexal neoplasm that manifests as a small, solitary, fleshcolored nodule usually in the head and neck region.4 Histologically, CL consists of well-circumscribed epithelial nests within the dermis that are peripherally outlined by palisading basaloid cells and filled with clear to eosinophilic epithelioid cells (Figure 2).5 The fibrotic tumor stroma often is infiltrated by numerous intralobular dendritic cells and lymphocytes that occasionally can be arranged in germinal center–like nodules.4 The lymphoepithelial nature of CL can be challenging to distinguish morphologically from LELC, and immunohistochemistry stains may be required. In CL, both the basaloid and epithelioid cells stain positive for pancytokeratin AE1/ AE3, but the peripheral palisaded basaloid cells also stain positive for BerEP4. Additionally, the fibrotic stroma can be highlighted by CD34 and the intralobular dendritic cells by S-100.4

Nasopharyngeal carcinoma (NPC), formerly known as lymphoepithelioma, refers to carcinoma arising within the epithelium of the nasopharynx.6 Endemic to China, NPC manifests as an enlarging nasopharyngeal mass, causing clinical symptoms such as nasal obstruction and epistaxis.7 Histologically, nonkeratinizing NPC exhibits a biphasic morphology consisting of epithelioid neoplastic cells and background lymphocytic infiltrates (Figure 3). The epithelial component consists of round to oval neoplastic cells with amphophilic to eosinophilic cytoplasm, vesicular nuclei, and prominent nucleoli.6 Nasopharyngeal carcinoma is associated strongly with the Epstein-Barr virus while LELC is not; thus, Epstein- Barr encoding region in situ hybridization can reliably distinguish these entities. Metastatic NPC is rare but has been reported; therefore, it is highly recommended to perform an otolaryngologic examination in addition to testing for Epstein-Barr virus reactivity as part of a complete evaluation.8

Cutaneous squamous cell carcinoma (SCC) is a common epidermal malignancy with multiple subtypes and variable morphology. The clinical presentation of SCC is similar to LELC—an enlarging hyperkeratotic papule or nodule on sun-exposed skin that often is ulcerated and tender.9 Histologically, poorly differentiated nonkeratinizing SCC can form nests and trabeculae of epithelioid cells that are stained by epithelial differentiation markers, resembling the epithelioid nests of LELC. Distinguishing between LELC and poorly differentiated SCC with robust inflammatory infiltrate can be challenging (Figure 4). In fact, some experts support LELC as an SCC variant rather than a separate entity.9 However, in contrast to LELC, the dermal nests of SCC usually maintain an epidermal connection and often are associated with an overlying area of SCC in situ or welldifferentiated SCC.3

Mycosis fungoides (MF) is a primary cutaneous T-cell lymphoma. It is the most common type of cutaneous lymphoma, accounting for almost 50% of all reported cases.10 Classic MF has an indolent course and progresses through several clinical stages. Patches and plaques characterize early stages; lymphadenopathy indicates progression to later stages in which erythroderma may develop with coalescence of patches, plaques, and tumors; and MF present in blood or lymph nodes characterizes the late stage. Each stage of MF is different histologically—from a superficial lichenoid infiltrate with exocytosis of malignant T cells in the patch stage, to more robust epidermotropism and dermal infiltrate in the plaque stage, and finally a dense dermal infiltrate in the late stage.11 The rare syringotropic variant of MF clinically manifests as solitary or multiple erythematous lesions, often with overlying alopecia. Syringotropic MF uniquely exhibits folliculotropism and syringotropism along with syringometaplasia on histologic evaluation (Figure 5).12 The syringometaplasia can be difficult to distinguish from the epithelial nests of LELC, particularly with the lymphocytic background. Immunohistochemical panels for T-cell markers can highlight aberrant T cells in syringotropic MF through their usual loss of CD5 and CD7, in comparison to normal T cells in LELC.11 An elevated CD4:CD8 ratio of 4:1 and molecular analysis for T-cell receptor gene clonal rearrangements also can support the diagnosis of MF.12

The Diagnosis: Lymphoepithelioma-like Carcinoma
Lymphoepithelioma-like carcinoma (LELC) is a rare, poorly differentiated, primary cutaneous neoplasm that occurs on sun-exposed skin, particularly on the head and neck of elderly individuals. It often manifests as an asymptomatic, slow-growing, flesh-colored or erythematous dermal nodule, though ulceration and tenderness have been reported.1 Histopathologically, these neoplasms often are poorly circumscribed and can infiltrate surrounding subcutaneous and soft tissue. As a biphasic tumor, LELC is characterized by islands, nests, or trabeculae of epithelioid cells within the mid dermis surrounded by a dense lymphocytic infiltrate with plasma cells (Figure 1).1 The epithelial component rarely communicates with the overlying epidermis and is composed of atypical polygonal cells with eosinophilic cytoplasm, vesicular nuclei, prominent nucleoli, and frequent mitosis.2 These epithelial nests can be highlighted by pancytokeratin AE1/AE3 or other epithelial differentiation markers (eg, CAM 5.2, CK5/6, epithelial membrane antigen, high-molecular-weight cytokeratin), while the surrounding lymphocytic infiltrate consists of an admixture of T cells and B cells. Lymphoepithelioma-like carcinomas also can demonstrate sebaceous, eccrine, or follicular differentiations.3 The epithelial nests of LELC also are positive for p63 and epithelial membrane antigen.2

The usual treatment of LELC is wide local excision or Mohs micrographic surgery.1 Despite the poorly differentiated morphology of the tumor, LELC has a generally good prognosis with low metastatic potential and few reports of local recurrence after incomplete excision.3 Patients who are not candidates for surgery as well as recalcitrant cases are managed with radiotherapy.1
Cutaneous lymphadenoma (CL) is a benign adnexal neoplasm that manifests as a small, solitary, fleshcolored nodule usually in the head and neck region.4 Histologically, CL consists of well-circumscribed epithelial nests within the dermis that are peripherally outlined by palisading basaloid cells and filled with clear to eosinophilic epithelioid cells (Figure 2).5 The fibrotic tumor stroma often is infiltrated by numerous intralobular dendritic cells and lymphocytes that occasionally can be arranged in germinal center–like nodules.4 The lymphoepithelial nature of CL can be challenging to distinguish morphologically from LELC, and immunohistochemistry stains may be required. In CL, both the basaloid and epithelioid cells stain positive for pancytokeratin AE1/ AE3, but the peripheral palisaded basaloid cells also stain positive for BerEP4. Additionally, the fibrotic stroma can be highlighted by CD34 and the intralobular dendritic cells by S-100.4

Nasopharyngeal carcinoma (NPC), formerly known as lymphoepithelioma, refers to carcinoma arising within the epithelium of the nasopharynx.6 Endemic to China, NPC manifests as an enlarging nasopharyngeal mass, causing clinical symptoms such as nasal obstruction and epistaxis.7 Histologically, nonkeratinizing NPC exhibits a biphasic morphology consisting of epithelioid neoplastic cells and background lymphocytic infiltrates (Figure 3). The epithelial component consists of round to oval neoplastic cells with amphophilic to eosinophilic cytoplasm, vesicular nuclei, and prominent nucleoli.6 Nasopharyngeal carcinoma is associated strongly with the Epstein-Barr virus while LELC is not; thus, Epstein- Barr encoding region in situ hybridization can reliably distinguish these entities. Metastatic NPC is rare but has been reported; therefore, it is highly recommended to perform an otolaryngologic examination in addition to testing for Epstein-Barr virus reactivity as part of a complete evaluation.8

Cutaneous squamous cell carcinoma (SCC) is a common epidermal malignancy with multiple subtypes and variable morphology. The clinical presentation of SCC is similar to LELC—an enlarging hyperkeratotic papule or nodule on sun-exposed skin that often is ulcerated and tender.9 Histologically, poorly differentiated nonkeratinizing SCC can form nests and trabeculae of epithelioid cells that are stained by epithelial differentiation markers, resembling the epithelioid nests of LELC. Distinguishing between LELC and poorly differentiated SCC with robust inflammatory infiltrate can be challenging (Figure 4). In fact, some experts support LELC as an SCC variant rather than a separate entity.9 However, in contrast to LELC, the dermal nests of SCC usually maintain an epidermal connection and often are associated with an overlying area of SCC in situ or welldifferentiated SCC.3

Mycosis fungoides (MF) is a primary cutaneous T-cell lymphoma. It is the most common type of cutaneous lymphoma, accounting for almost 50% of all reported cases.10 Classic MF has an indolent course and progresses through several clinical stages. Patches and plaques characterize early stages; lymphadenopathy indicates progression to later stages in which erythroderma may develop with coalescence of patches, plaques, and tumors; and MF present in blood or lymph nodes characterizes the late stage. Each stage of MF is different histologically—from a superficial lichenoid infiltrate with exocytosis of malignant T cells in the patch stage, to more robust epidermotropism and dermal infiltrate in the plaque stage, and finally a dense dermal infiltrate in the late stage.11 The rare syringotropic variant of MF clinically manifests as solitary or multiple erythematous lesions, often with overlying alopecia. Syringotropic MF uniquely exhibits folliculotropism and syringotropism along with syringometaplasia on histologic evaluation (Figure 5).12 The syringometaplasia can be difficult to distinguish from the epithelial nests of LELC, particularly with the lymphocytic background. Immunohistochemical panels for T-cell markers can highlight aberrant T cells in syringotropic MF through their usual loss of CD5 and CD7, in comparison to normal T cells in LELC.11 An elevated CD4:CD8 ratio of 4:1 and molecular analysis for T-cell receptor gene clonal rearrangements also can support the diagnosis of MF.12

- Morteza Abedi S, Salama S, Alowami S. Lymphoepithelioma-like carcinoma of the skin: case report and approach to surgical pathology sign out. Rare Tumors. 2013;5:E47.
- Fisher JC, White RM, Hurd DS. Lymphoepithelioma-like carcinoma of the skin: a case of one patient presenting with two primary cutaneous neoplasms. J Am Osteopath Coll Dermatol. 2015;33:40-41.
- Welch PQ, Williams SB, Foss RD, et al. Lymphoepithelioma-like carcinoma of head and neck skin: a systematic analysis of 11 cases and review of literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011;111:78-86.
- Yu R, Salama S, Alowami S. Cutaneous lymphadenoma: a rare case and brief review of a diagnostic pitfall. Rare Tumors. 2014;6:5358.
- Monteagudo C, Fúnez R, Sánchez-Sendra B, et al. Cutaneous lymphadenoma is a distinct trichoblastoma-like lymphoepithelial tumor with diffuse androgen receptor immunoreactivity, Notch1 ligand in Reed-Sternberg-like Cells, and common EGFR somatic mutations. Am J Surg Pathol. 2021;45:1382-1390.
- Stelow EB, Wenig BM. Update from the 4th edition of the World Health Organization classification of head and neck tumours: nasopharynx. Head Neck Pathol. 2017;11:16-22.
- Almomani MH, Zulfiqar H, Nagalli S. Nasopharyngeal carcinoma (NPC, lymphoepithelioma). StatPearls Publishing; 2022.
- Lassen CB, Lock-Andersen J. Lymphoepithelioma-like carcinoma of the skin: a case with perineural invasion. Plast Reconstr Surg Glob Open. 2014;2:E252.
- Motaparthi K, Kapil JP, Velazquez EF. Cutaneous squamous cell carcinoma: review of the eighth edition of the American Joint Committee on Cancer Staging Guidelines, Prognostic Factors, and Histopathologic Variants. Adv Anat Pathol. 2017;24:171-194.
- Pileri A, Facchetti F, Rütten A, et al. Syringotropic mycosis fungoides: a rare variant of the disease with peculiar clinicopathologic features. Am J Surg Pathol. 2011;35:100-109.
- Ryu HJ, Kim SI, Jang HO, et al. Evaluation of the International Society for Cutaneous Lymphoma Algorithm for the Diagnosis of Early Mycosis Fungoides [published October 15, 2021]. Cells. 2021;10:2758. doi:10.3390/cells10102758
- Lehmer LM, Amber KT, de Feraudy SM. Syringotropic mycosis fungoides: a rare form of cutaneous T-cell lymphoma enabling a histopathologic “sigh of relief.” Am J Dermatopathol. 2017;39:920-923.
- Morteza Abedi S, Salama S, Alowami S. Lymphoepithelioma-like carcinoma of the skin: case report and approach to surgical pathology sign out. Rare Tumors. 2013;5:E47.
- Fisher JC, White RM, Hurd DS. Lymphoepithelioma-like carcinoma of the skin: a case of one patient presenting with two primary cutaneous neoplasms. J Am Osteopath Coll Dermatol. 2015;33:40-41.
- Welch PQ, Williams SB, Foss RD, et al. Lymphoepithelioma-like carcinoma of head and neck skin: a systematic analysis of 11 cases and review of literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011;111:78-86.
- Yu R, Salama S, Alowami S. Cutaneous lymphadenoma: a rare case and brief review of a diagnostic pitfall. Rare Tumors. 2014;6:5358.
- Monteagudo C, Fúnez R, Sánchez-Sendra B, et al. Cutaneous lymphadenoma is a distinct trichoblastoma-like lymphoepithelial tumor with diffuse androgen receptor immunoreactivity, Notch1 ligand in Reed-Sternberg-like Cells, and common EGFR somatic mutations. Am J Surg Pathol. 2021;45:1382-1390.
- Stelow EB, Wenig BM. Update from the 4th edition of the World Health Organization classification of head and neck tumours: nasopharynx. Head Neck Pathol. 2017;11:16-22.
- Almomani MH, Zulfiqar H, Nagalli S. Nasopharyngeal carcinoma (NPC, lymphoepithelioma). StatPearls Publishing; 2022.
- Lassen CB, Lock-Andersen J. Lymphoepithelioma-like carcinoma of the skin: a case with perineural invasion. Plast Reconstr Surg Glob Open. 2014;2:E252.
- Motaparthi K, Kapil JP, Velazquez EF. Cutaneous squamous cell carcinoma: review of the eighth edition of the American Joint Committee on Cancer Staging Guidelines, Prognostic Factors, and Histopathologic Variants. Adv Anat Pathol. 2017;24:171-194.
- Pileri A, Facchetti F, Rütten A, et al. Syringotropic mycosis fungoides: a rare variant of the disease with peculiar clinicopathologic features. Am J Surg Pathol. 2011;35:100-109.
- Ryu HJ, Kim SI, Jang HO, et al. Evaluation of the International Society for Cutaneous Lymphoma Algorithm for the Diagnosis of Early Mycosis Fungoides [published October 15, 2021]. Cells. 2021;10:2758. doi:10.3390/cells10102758
- Lehmer LM, Amber KT, de Feraudy SM. Syringotropic mycosis fungoides: a rare form of cutaneous T-cell lymphoma enabling a histopathologic “sigh of relief.” Am J Dermatopathol. 2017;39:920-923.
A 77-year-old man presented with a 1.2-cm dermal nodule on the left temple of 1 year’s duration. The lesion had become tender and darker in color. An excision was performed and submitted for histologic examination. Additional immunohistochemistry staining for Epstein-Barr virus was negative.
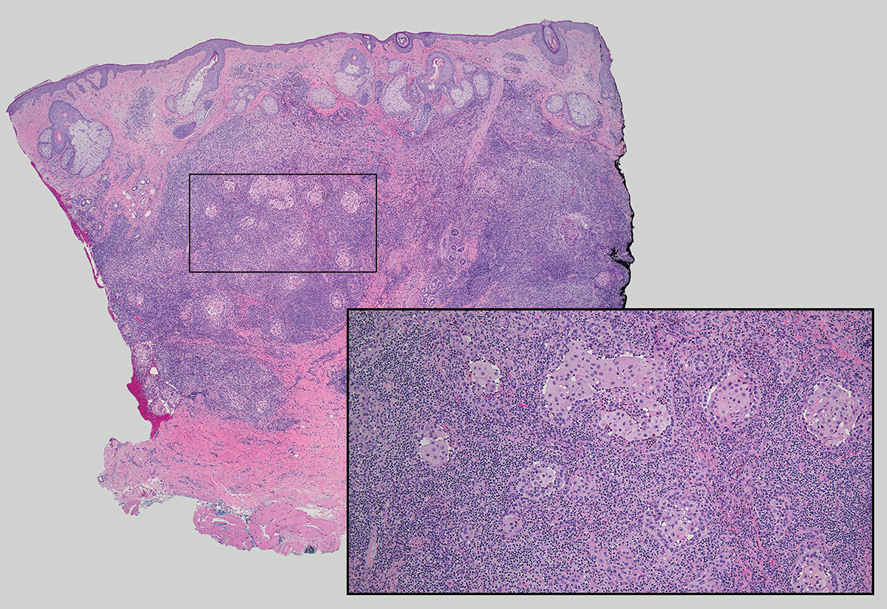
Obesity in Children
Attacks on Emergency Room Workers Prompt Debate Over Tougher Penalties
Patients hurl verbal abuse at Michelle Ravera every day in the emergency room. Physical violence is less common, she said, but has become a growing threat.
Ravera, an ER nurse at Sutter Medical Center in Sacramento, recalled an incident in which an agitated patient wanted to leave. “Without any warning he just reached up, grabbed my glasses, and punched me in the face,” said Ravera, 54. “And then he was getting ready to attack another patient in the room.” Ravera and hospital security guards subdued the patient so he couldn’t hurt anyone else.
Covid-19 only made things worse: With routine care harder to come by, many patients ended up in the ER with serious diseases — and brimming with frustrations.
In California, simple assault against workers inside an ER is considered the same as simple assault against almost anyone else, and carries a maximum punishment of a $1,000 fine and six months in jail. In contrast, simple assault against emergency medical workers in the field, such as an EMT responding to a 911 call, carries maximum penalties of a $2,000 fine and a year in jail. Simple assault does not involve the use of a deadly weapon or the intention to inflict serious bodily injury.
State Assembly member Freddie Rodriguez, who worked as an EMT, has authored a bill to make the punishments consistent: a $2,000 fine and one year in jail for simple assault on any on-the-job emergency health care worker, whether in the field or an ER. The measure would also eliminate the discrepancy for simple battery.
Patients and family members are assaulting staff and “doing things they shouldn’t be doing to the people that are there to take care of your loved ones,” said Rodriguez, a Democrat from Pomona. The bill passed the state Assembly unanimously in January and awaits consideration in the Senate.
Rodriguez has introduced similar measures twice before. Then-Gov. Jerry Brown vetoed one in 2015, saying he doubted a longer jail sentence would deter violence. “We need to find more creative ways to protect the safety of these critical workers,” he wrote in his veto message. The 2019 bill died in the state Senate.
Rodriguez said ERs have become more dangerous for health care workers since then and that “there has to be accountability” for violent behavior. Opponents fear stiffer penalties would be levied disproportionately on patients of color or those with developmental disabilities. They also point out that violent patients can already face penalties under existing assault and battery laws.
Data from the California Division of Occupational Safety and Health shows that reported attacks on ER workers by patients, visitors, and strangers jumped about 25% from 2018 to 2023, from 2,587 to 3,238. The rate of attacks per 100,000 ER visits also increased.
Punching, kicking, pushing, and similar aggression accounted for most of the attacks. Only a small number included weapons.
These numbers are likely an undercount, said Al’ai Alvarez, an ER doctor and clinical associate professor at Stanford University’s Department of Emergency Medicine. Many hospital staffers don’t fill out workplace violence reports because they don’t have time or feel nothing will come of it, he said.
Ravera remembers when her community rallied around health care workers at the start of the pandemic, acting respectfully and bringing food and extra N95 masks to workers.
“Then something just switched,” she said. “The patients became angrier and more aggressive.”
Violence can contribute to burnout and drive workers to quit — or worse, said Alvarez, who has lost colleagues to suicide, and thinks burnout was a key factor. “The cost of burnout is more than just loss of productivity,” he said. “It’s loss of human beings that also had the potential to take care of many more people.”
The National Center for Health Workforce Analysis projects California will experience an 18% shortage of all types of nurses in 2035, the third worst in the country.
Federal legislation called the Safety From Violence for Healthcare Employees Act would set sentences of up to 10 years for assault against a health care worker, not limited to emergency workers, and up to 20 years in cases involving dangerous weapons or bodily injury. Though it was introduced in 2023, it has not yet had a committee hearing.
Opponents of the California bill, which include ACLU California Action, the California Public Defenders Association, and advocates for people with autism, argue it wouldn’t deter attacks — and would unfairly target certain patients.
“There’s no evidence to suggest that increased penalties are going to meaningfully address this conduct,” said Eric Henderson, a legislative advocate for ACLU California Action. “Most importantly, there are already laws on the books to address assaultive conduct.”
Beth Burt, executive director of the Autism Society Inland Empire, said the measure doesn’t take into account the special needs of people with autism and other developmental disorders.
The smells, lights, textures, and crowds in the ER can overstimulate a person with autism, she said. When that happens, they can struggle to articulate their feelings, which can result in a violent outburst, “whether it’s a 9-year-old or a 29-year-old,” Burt said.
She worries that hospital staff may misunderstand these reactions, and involve law enforcement when it’s not necessary. As “a parent, it is still my worst fear” that she’ll get a phone call to inform her that her adult son with autism has been arrested, she said.
Burt would rather the state prioritize de-escalation programs over penalties, such as the training programs for first responders she helped create through the Autism Society Inland Empire. After implementing the training, hospital administrators asked Burt to share some strategies with them, she said. Hospital security staffers who do not want to use physical restraints on autistic patients have also sought her advice, she said.
Supporters of the bill, including health care and law enforcement groups, counter that people with mental health conditions or autism who are charged with assault in an ER may be eligible for existing programs that provide mental health treatment in lieu of a criminal sentence.
Stephanie Jensen, an ER nurse and head of governmental affairs for the Emergency Nurses Association, California State Council, said her organization is simply arguing for equity. “If you punch me in the hospital, it’s the same as if you punch me on the street,” she said.
If lawmakers don’t act, she warned, there won’t be enough workers for the patients who need them.
“It’s hard to keep those human resources accessible when it just seems like you’re showing up to get beat up every day,” Jensen said. “The emergency department is taking it on the chin, literally and figuratively.”
This article was produced by KFF Health News, which publishes California Healthline, an editorially independent service of the California Health Care Foundation. KFF Health News is a national newsroom that produces in-depth journalism about health issues and is one of the core operating programs at KFF—an independent source of health policy research, polling, and journalism. Learn more about KFF.
Patients hurl verbal abuse at Michelle Ravera every day in the emergency room. Physical violence is less common, she said, but has become a growing threat.
Ravera, an ER nurse at Sutter Medical Center in Sacramento, recalled an incident in which an agitated patient wanted to leave. “Without any warning he just reached up, grabbed my glasses, and punched me in the face,” said Ravera, 54. “And then he was getting ready to attack another patient in the room.” Ravera and hospital security guards subdued the patient so he couldn’t hurt anyone else.
Covid-19 only made things worse: With routine care harder to come by, many patients ended up in the ER with serious diseases — and brimming with frustrations.
In California, simple assault against workers inside an ER is considered the same as simple assault against almost anyone else, and carries a maximum punishment of a $1,000 fine and six months in jail. In contrast, simple assault against emergency medical workers in the field, such as an EMT responding to a 911 call, carries maximum penalties of a $2,000 fine and a year in jail. Simple assault does not involve the use of a deadly weapon or the intention to inflict serious bodily injury.
State Assembly member Freddie Rodriguez, who worked as an EMT, has authored a bill to make the punishments consistent: a $2,000 fine and one year in jail for simple assault on any on-the-job emergency health care worker, whether in the field or an ER. The measure would also eliminate the discrepancy for simple battery.
Patients and family members are assaulting staff and “doing things they shouldn’t be doing to the people that are there to take care of your loved ones,” said Rodriguez, a Democrat from Pomona. The bill passed the state Assembly unanimously in January and awaits consideration in the Senate.
Rodriguez has introduced similar measures twice before. Then-Gov. Jerry Brown vetoed one in 2015, saying he doubted a longer jail sentence would deter violence. “We need to find more creative ways to protect the safety of these critical workers,” he wrote in his veto message. The 2019 bill died in the state Senate.
Rodriguez said ERs have become more dangerous for health care workers since then and that “there has to be accountability” for violent behavior. Opponents fear stiffer penalties would be levied disproportionately on patients of color or those with developmental disabilities. They also point out that violent patients can already face penalties under existing assault and battery laws.
Data from the California Division of Occupational Safety and Health shows that reported attacks on ER workers by patients, visitors, and strangers jumped about 25% from 2018 to 2023, from 2,587 to 3,238. The rate of attacks per 100,000 ER visits also increased.
Punching, kicking, pushing, and similar aggression accounted for most of the attacks. Only a small number included weapons.
These numbers are likely an undercount, said Al’ai Alvarez, an ER doctor and clinical associate professor at Stanford University’s Department of Emergency Medicine. Many hospital staffers don’t fill out workplace violence reports because they don’t have time or feel nothing will come of it, he said.
Ravera remembers when her community rallied around health care workers at the start of the pandemic, acting respectfully and bringing food and extra N95 masks to workers.
“Then something just switched,” she said. “The patients became angrier and more aggressive.”
Violence can contribute to burnout and drive workers to quit — or worse, said Alvarez, who has lost colleagues to suicide, and thinks burnout was a key factor. “The cost of burnout is more than just loss of productivity,” he said. “It’s loss of human beings that also had the potential to take care of many more people.”
The National Center for Health Workforce Analysis projects California will experience an 18% shortage of all types of nurses in 2035, the third worst in the country.
Federal legislation called the Safety From Violence for Healthcare Employees Act would set sentences of up to 10 years for assault against a health care worker, not limited to emergency workers, and up to 20 years in cases involving dangerous weapons or bodily injury. Though it was introduced in 2023, it has not yet had a committee hearing.
Opponents of the California bill, which include ACLU California Action, the California Public Defenders Association, and advocates for people with autism, argue it wouldn’t deter attacks — and would unfairly target certain patients.
“There’s no evidence to suggest that increased penalties are going to meaningfully address this conduct,” said Eric Henderson, a legislative advocate for ACLU California Action. “Most importantly, there are already laws on the books to address assaultive conduct.”
Beth Burt, executive director of the Autism Society Inland Empire, said the measure doesn’t take into account the special needs of people with autism and other developmental disorders.
The smells, lights, textures, and crowds in the ER can overstimulate a person with autism, she said. When that happens, they can struggle to articulate their feelings, which can result in a violent outburst, “whether it’s a 9-year-old or a 29-year-old,” Burt said.
She worries that hospital staff may misunderstand these reactions, and involve law enforcement when it’s not necessary. As “a parent, it is still my worst fear” that she’ll get a phone call to inform her that her adult son with autism has been arrested, she said.
Burt would rather the state prioritize de-escalation programs over penalties, such as the training programs for first responders she helped create through the Autism Society Inland Empire. After implementing the training, hospital administrators asked Burt to share some strategies with them, she said. Hospital security staffers who do not want to use physical restraints on autistic patients have also sought her advice, she said.
Supporters of the bill, including health care and law enforcement groups, counter that people with mental health conditions or autism who are charged with assault in an ER may be eligible for existing programs that provide mental health treatment in lieu of a criminal sentence.
Stephanie Jensen, an ER nurse and head of governmental affairs for the Emergency Nurses Association, California State Council, said her organization is simply arguing for equity. “If you punch me in the hospital, it’s the same as if you punch me on the street,” she said.
If lawmakers don’t act, she warned, there won’t be enough workers for the patients who need them.
“It’s hard to keep those human resources accessible when it just seems like you’re showing up to get beat up every day,” Jensen said. “The emergency department is taking it on the chin, literally and figuratively.”
This article was produced by KFF Health News, which publishes California Healthline, an editorially independent service of the California Health Care Foundation. KFF Health News is a national newsroom that produces in-depth journalism about health issues and is one of the core operating programs at KFF—an independent source of health policy research, polling, and journalism. Learn more about KFF.
Patients hurl verbal abuse at Michelle Ravera every day in the emergency room. Physical violence is less common, she said, but has become a growing threat.
Ravera, an ER nurse at Sutter Medical Center in Sacramento, recalled an incident in which an agitated patient wanted to leave. “Without any warning he just reached up, grabbed my glasses, and punched me in the face,” said Ravera, 54. “And then he was getting ready to attack another patient in the room.” Ravera and hospital security guards subdued the patient so he couldn’t hurt anyone else.
Covid-19 only made things worse: With routine care harder to come by, many patients ended up in the ER with serious diseases — and brimming with frustrations.
In California, simple assault against workers inside an ER is considered the same as simple assault against almost anyone else, and carries a maximum punishment of a $1,000 fine and six months in jail. In contrast, simple assault against emergency medical workers in the field, such as an EMT responding to a 911 call, carries maximum penalties of a $2,000 fine and a year in jail. Simple assault does not involve the use of a deadly weapon or the intention to inflict serious bodily injury.
State Assembly member Freddie Rodriguez, who worked as an EMT, has authored a bill to make the punishments consistent: a $2,000 fine and one year in jail for simple assault on any on-the-job emergency health care worker, whether in the field or an ER. The measure would also eliminate the discrepancy for simple battery.
Patients and family members are assaulting staff and “doing things they shouldn’t be doing to the people that are there to take care of your loved ones,” said Rodriguez, a Democrat from Pomona. The bill passed the state Assembly unanimously in January and awaits consideration in the Senate.
Rodriguez has introduced similar measures twice before. Then-Gov. Jerry Brown vetoed one in 2015, saying he doubted a longer jail sentence would deter violence. “We need to find more creative ways to protect the safety of these critical workers,” he wrote in his veto message. The 2019 bill died in the state Senate.
Rodriguez said ERs have become more dangerous for health care workers since then and that “there has to be accountability” for violent behavior. Opponents fear stiffer penalties would be levied disproportionately on patients of color or those with developmental disabilities. They also point out that violent patients can already face penalties under existing assault and battery laws.
Data from the California Division of Occupational Safety and Health shows that reported attacks on ER workers by patients, visitors, and strangers jumped about 25% from 2018 to 2023, from 2,587 to 3,238. The rate of attacks per 100,000 ER visits also increased.
Punching, kicking, pushing, and similar aggression accounted for most of the attacks. Only a small number included weapons.
These numbers are likely an undercount, said Al’ai Alvarez, an ER doctor and clinical associate professor at Stanford University’s Department of Emergency Medicine. Many hospital staffers don’t fill out workplace violence reports because they don’t have time or feel nothing will come of it, he said.
Ravera remembers when her community rallied around health care workers at the start of the pandemic, acting respectfully and bringing food and extra N95 masks to workers.
“Then something just switched,” she said. “The patients became angrier and more aggressive.”
Violence can contribute to burnout and drive workers to quit — or worse, said Alvarez, who has lost colleagues to suicide, and thinks burnout was a key factor. “The cost of burnout is more than just loss of productivity,” he said. “It’s loss of human beings that also had the potential to take care of many more people.”
The National Center for Health Workforce Analysis projects California will experience an 18% shortage of all types of nurses in 2035, the third worst in the country.
Federal legislation called the Safety From Violence for Healthcare Employees Act would set sentences of up to 10 years for assault against a health care worker, not limited to emergency workers, and up to 20 years in cases involving dangerous weapons or bodily injury. Though it was introduced in 2023, it has not yet had a committee hearing.
Opponents of the California bill, which include ACLU California Action, the California Public Defenders Association, and advocates for people with autism, argue it wouldn’t deter attacks — and would unfairly target certain patients.
“There’s no evidence to suggest that increased penalties are going to meaningfully address this conduct,” said Eric Henderson, a legislative advocate for ACLU California Action. “Most importantly, there are already laws on the books to address assaultive conduct.”
Beth Burt, executive director of the Autism Society Inland Empire, said the measure doesn’t take into account the special needs of people with autism and other developmental disorders.
The smells, lights, textures, and crowds in the ER can overstimulate a person with autism, she said. When that happens, they can struggle to articulate their feelings, which can result in a violent outburst, “whether it’s a 9-year-old or a 29-year-old,” Burt said.
She worries that hospital staff may misunderstand these reactions, and involve law enforcement when it’s not necessary. As “a parent, it is still my worst fear” that she’ll get a phone call to inform her that her adult son with autism has been arrested, she said.
Burt would rather the state prioritize de-escalation programs over penalties, such as the training programs for first responders she helped create through the Autism Society Inland Empire. After implementing the training, hospital administrators asked Burt to share some strategies with them, she said. Hospital security staffers who do not want to use physical restraints on autistic patients have also sought her advice, she said.
Supporters of the bill, including health care and law enforcement groups, counter that people with mental health conditions or autism who are charged with assault in an ER may be eligible for existing programs that provide mental health treatment in lieu of a criminal sentence.
Stephanie Jensen, an ER nurse and head of governmental affairs for the Emergency Nurses Association, California State Council, said her organization is simply arguing for equity. “If you punch me in the hospital, it’s the same as if you punch me on the street,” she said.
If lawmakers don’t act, she warned, there won’t be enough workers for the patients who need them.
“It’s hard to keep those human resources accessible when it just seems like you’re showing up to get beat up every day,” Jensen said. “The emergency department is taking it on the chin, literally and figuratively.”
This article was produced by KFF Health News, which publishes California Healthline, an editorially independent service of the California Health Care Foundation. KFF Health News is a national newsroom that produces in-depth journalism about health issues and is one of the core operating programs at KFF—an independent source of health policy research, polling, and journalism. Learn more about KFF.
Lead Has Not Gone Away — What Should Pediatric Clinicians Do?
following a 2023 outbreak of elevated levels of lead in children associated with consumption of contaminated applesauce.
Federal legislation in the 1970s eliminated lead from gasoline, paints, and other consumer products, and resulted in significantly reduced blood lead levels (BLLs) in children throughout the United States.
But recently published studies highlight persistent issues with lead in drinking water and consumer products, suggesting that the fight is not over.
It’s in the Water
In 2014 the city of Flint, Michigan, changed its water supply and high levels of lead were later found in the municipal water supply.
Effects of that crisis still plague the city today. An initial study found that elevated BLLs had doubled among children between 2013 and 2015.
Lead exposure in young children is associated with several negative outcomes, including decreased cognitive ability, brain volume, and social mobility, and increased anxiety/depression and impulsivity, and higher rates of criminal offenses later in life.
Many other water systems still contain lead pipes, despite a 1986 ban by the US Environmental Protection Agency on using them for installing or repairing public water systems. The mayor of Chicago announced a plan to start replacing lead service lines in 2020; however, 400,000 households are still served by these pipes, the most in the nation.
Benjamin Huynh, a native of Chicago, was curious about the impact of all those lead service lines. Now an assistant professor in the Department of Environmental Health and Engineering at Johns Hopkins University in Baltimore, Maryland, he and his colleagues researched how many children under the age of 6 years were exposed to contaminated water.
The results showed that lead contamination of water is widespread.
“We’re estimating that 68% of kids under the age of 6 in Chicago were exposed to lead-contaminated drinking water,” Mr. Huynh said.
He added that residents in predominantly Black and Latino neighborhoods had the highest risk for lead contamination in their water, but children living on these blocks were less likely to get tested, suggesting a need for more outreach to raise awareness.
Meanwhile, a little over one third of Chicago residents reported drinking bottled water as their main source of drinking water.
But even bottled water could contain lead. The US Food and Drug Administration (FDA) has set a limit for lead in bottled water to five parts per billion. The FDA threshold for taking action in public drinking water systems is 15 parts per billion. But the American Academy of Pediatrics states that no amount of lead in drinking water is considered safe for drinking.
Mr. Huynh also pointed out that not all home water filters remove lead. Only devices that meet National Sanitation Foundation 53 standards are certified for lead removal. Consumers should verify that the filter package specifically lists the device as certified for removing contaminant lead.
Lead-tainted Cinnamon
Last fall, the North Carolina Department of Health and Human Services identified several children with elevated levels of lead who had consumed WanaBana Apple Cinnamon Fruit Puree pouches.
An investigation by the FDA identified additional brands containing lead and issued a recall of applesauce pouches sold by retailers like Dollar Tree and Amazon.
According to the US Centers for Disease Control and Prevention, nearly 500 children were affected by the tainted applesauce. The FDA traced the source of the lead to cinnamon from a supplier in Ecuador.
An FDA spokesperson told this news organization the episode appears to have resulted from “economically motivated adulteration,” which occurs when a manufacturer leaves out or substitutes a valuable ingredient or part of a food. In the case of spices, lead may be added as a coloring agent or to increase the product weight.
“When we look at domestically made products from large, reputable companies, in general, they do a pretty good job of following safe product guidelines and regulations,” said Kevin Osterhoudt, MD, professor of pediatrics at the Perelman School of Medicine at the University of Pennsylvania in Philadelphia. “But when we use third-party sellers and we import things from other countries that aren’t regulated as closely, we certainly take a lot more risk in the products that we receive.”
While the Food Safety Modernization Act of 2011 aimed to improve agency’s capacity to manage the ever-rising volume of food produced domestically and imported from overseas, the funding has stayed flat while the volume of inspections has increased. In the early 1990s, the number of shipments screened by the agency numbered in the thousands annually. Last year the FDA screened 15 million shipments from more than 200 countries, according to the agency.
Prompted by the finding of lead in applesauce, the FDA began a wider investigation into ground cinnamon by sampling the product from discount retail stores. It recalled an additional six brands of cinnamon sold in the United States containing lead.
Dr. Osterhoudt’s message to families who think their child might have been exposed to a contaminated product is to dispose of it as directed by FDA and CDC guidelines.
In Philadelphia, where Dr. Osterhoudt practices as an emergency room physician, baseline rates of childhood lead poisoning are already high, so he advises families to “do a larger inventory of all the source potential sources of lead in their life and to reduce all the exposures as low as possible.”
He also advises parents that a nutritious diet high in calcium and iron can protect their children from the deleterious effects of lead.
Current Standards for Lead Screening and Testing
Lead is ubiquitous. The common routes of exposure to humans include use of fossil fuels such as leaded gasoline, some types of industrial facilities, and past use of lead-based paint in homes. In addition to spices, lead has been found in a wide variety of products such as toys, jewelry, antiques, cosmetics, and dietary supplements imported from other countries.
Noah Buncher, DO, is a primary care pediatrician in South Philadelphia at Children’s Hospital of Pennsylvania and the former director of a lead clinic in Boston that provides care for children with lead poisoning. He follows guidelines from the American Academy of Pediatrics that define an elevated BLL as ≥ 3.5 µg/dL. The guidelines recommend screening children for lead exposures during well child visits starting at age 6 months up to 6 years and obtaining a BLL if risks for lead exposure are present.
Dr. Buncher starts with a basic environmental history that covers items like the age, condition, zip code of home, parental occupations, or hobbies that might result in exposing family members to lead, and if another child in the home has a history of elevated BLLs.
But a careful history for potential lead exposures can be time-consuming.
“There’s a lot to cover in a routine well child visit,” Dr. Buncher said. “We have maybe 15-20 minutes to cover a lot.”
Clinics also vary on whether lead screening questions are put into workflows in the electronic medical record. Although parents can complete a written questionnaire about possible lead exposures, they may have difficulty answering questions about the age of their home or not know whether their occupation is high risk.
Transportation to a clinic is often a barrier for families, and sometimes patients must travel to a separate lab to be tested for lead.
Dr. Buncher also pointed to the patchwork of local and state requirements that can lead to confusion among providers. Massachusetts, where he formerly practiced, has a universal requirement to test all children at ages 1, 2, and 3 years. But in Pennsylvania, screening laws vary from county to county.
“Pennsylvania should implement universal screening recommendations for all kids under 6 regardless of what county you live in,” Dr. Buncher said.
Protective Measures
Alan Woolf, MD, a professor of pediatrics at Harvard Medical School, Boston, Massachusetts, and director of the Pediatric Environmental Health Center at Boston Children’s Hospital, has a few ideas about how providers can step up their lead game, including partnering with their local health department.
The CDC funds Childhood Lead Poisoning Prevention Programs based in state and local health departments to work with clinicians to improve rates of blood lead testing, monitor the prevalence of lead in their jurisdictions, and ensure that a system of referral is available for treatment and lead remediation services in the home.
Dr. Woolf also suggested that clinicians refer patients under age 3 years with high BLLs to their local Early Intervention Program.
“They’ll assess their child’s development, their speech, their motor skills, their social skills, and if they qualify, it’s free,” Dr. Woolf said.
He cited research showing children with elevated lead levels who received early intervention services performed better in grade school than equally exposed children who did not access similar services.
Another key strategy for pediatric clinicians is to learn local or state regulations for testing children for lead and how to access lead surveillance data in their practice area. Children who reside in high-risk areas are automatic candidates for screening.
Dr. Woolf pointed out that big cities are not the only localities with lead in the drinking water. If families are drawing water from their own well, they should collect that water annually to have it tested for lead and microbes.
At the clinic-wide level, Dr. Woolf recommends the use of blood lead testing as a quality improvement measure. For example, Akron Children’s Hospital developed a quality improvement initiative using a clinical decision support tool to raise screening rates in their network of 30 clinics. One year after beginning the project, lead screenings during 12-month well visits increased from 71% to 96%.
“What we’re interested in as pediatric health professionals is eliminating all background sources of lead in a child’s environment,” Dr. Woolf said. “Whether that’s applesauce pouches, whether that’s lead-containing paint, lead in water, lead in spices, or lead in imported pottery or cookware — there are just a tremendous number of sources of lead that we can do something about.”
None of the subjects reported financial conflicts of interest.
A former pediatrician, Dr. Thomas is a freelance science writer living in Portland, Oregon.
A version of this article appeared on Medscape.com.
following a 2023 outbreak of elevated levels of lead in children associated with consumption of contaminated applesauce.
Federal legislation in the 1970s eliminated lead from gasoline, paints, and other consumer products, and resulted in significantly reduced blood lead levels (BLLs) in children throughout the United States.
But recently published studies highlight persistent issues with lead in drinking water and consumer products, suggesting that the fight is not over.
It’s in the Water
In 2014 the city of Flint, Michigan, changed its water supply and high levels of lead were later found in the municipal water supply.
Effects of that crisis still plague the city today. An initial study found that elevated BLLs had doubled among children between 2013 and 2015.
Lead exposure in young children is associated with several negative outcomes, including decreased cognitive ability, brain volume, and social mobility, and increased anxiety/depression and impulsivity, and higher rates of criminal offenses later in life.
Many other water systems still contain lead pipes, despite a 1986 ban by the US Environmental Protection Agency on using them for installing or repairing public water systems. The mayor of Chicago announced a plan to start replacing lead service lines in 2020; however, 400,000 households are still served by these pipes, the most in the nation.
Benjamin Huynh, a native of Chicago, was curious about the impact of all those lead service lines. Now an assistant professor in the Department of Environmental Health and Engineering at Johns Hopkins University in Baltimore, Maryland, he and his colleagues researched how many children under the age of 6 years were exposed to contaminated water.
The results showed that lead contamination of water is widespread.
“We’re estimating that 68% of kids under the age of 6 in Chicago were exposed to lead-contaminated drinking water,” Mr. Huynh said.
He added that residents in predominantly Black and Latino neighborhoods had the highest risk for lead contamination in their water, but children living on these blocks were less likely to get tested, suggesting a need for more outreach to raise awareness.
Meanwhile, a little over one third of Chicago residents reported drinking bottled water as their main source of drinking water.
But even bottled water could contain lead. The US Food and Drug Administration (FDA) has set a limit for lead in bottled water to five parts per billion. The FDA threshold for taking action in public drinking water systems is 15 parts per billion. But the American Academy of Pediatrics states that no amount of lead in drinking water is considered safe for drinking.
Mr. Huynh also pointed out that not all home water filters remove lead. Only devices that meet National Sanitation Foundation 53 standards are certified for lead removal. Consumers should verify that the filter package specifically lists the device as certified for removing contaminant lead.
Lead-tainted Cinnamon
Last fall, the North Carolina Department of Health and Human Services identified several children with elevated levels of lead who had consumed WanaBana Apple Cinnamon Fruit Puree pouches.
An investigation by the FDA identified additional brands containing lead and issued a recall of applesauce pouches sold by retailers like Dollar Tree and Amazon.
According to the US Centers for Disease Control and Prevention, nearly 500 children were affected by the tainted applesauce. The FDA traced the source of the lead to cinnamon from a supplier in Ecuador.
An FDA spokesperson told this news organization the episode appears to have resulted from “economically motivated adulteration,” which occurs when a manufacturer leaves out or substitutes a valuable ingredient or part of a food. In the case of spices, lead may be added as a coloring agent or to increase the product weight.
“When we look at domestically made products from large, reputable companies, in general, they do a pretty good job of following safe product guidelines and regulations,” said Kevin Osterhoudt, MD, professor of pediatrics at the Perelman School of Medicine at the University of Pennsylvania in Philadelphia. “But when we use third-party sellers and we import things from other countries that aren’t regulated as closely, we certainly take a lot more risk in the products that we receive.”
While the Food Safety Modernization Act of 2011 aimed to improve agency’s capacity to manage the ever-rising volume of food produced domestically and imported from overseas, the funding has stayed flat while the volume of inspections has increased. In the early 1990s, the number of shipments screened by the agency numbered in the thousands annually. Last year the FDA screened 15 million shipments from more than 200 countries, according to the agency.
Prompted by the finding of lead in applesauce, the FDA began a wider investigation into ground cinnamon by sampling the product from discount retail stores. It recalled an additional six brands of cinnamon sold in the United States containing lead.
Dr. Osterhoudt’s message to families who think their child might have been exposed to a contaminated product is to dispose of it as directed by FDA and CDC guidelines.
In Philadelphia, where Dr. Osterhoudt practices as an emergency room physician, baseline rates of childhood lead poisoning are already high, so he advises families to “do a larger inventory of all the source potential sources of lead in their life and to reduce all the exposures as low as possible.”
He also advises parents that a nutritious diet high in calcium and iron can protect their children from the deleterious effects of lead.
Current Standards for Lead Screening and Testing
Lead is ubiquitous. The common routes of exposure to humans include use of fossil fuels such as leaded gasoline, some types of industrial facilities, and past use of lead-based paint in homes. In addition to spices, lead has been found in a wide variety of products such as toys, jewelry, antiques, cosmetics, and dietary supplements imported from other countries.
Noah Buncher, DO, is a primary care pediatrician in South Philadelphia at Children’s Hospital of Pennsylvania and the former director of a lead clinic in Boston that provides care for children with lead poisoning. He follows guidelines from the American Academy of Pediatrics that define an elevated BLL as ≥ 3.5 µg/dL. The guidelines recommend screening children for lead exposures during well child visits starting at age 6 months up to 6 years and obtaining a BLL if risks for lead exposure are present.
Dr. Buncher starts with a basic environmental history that covers items like the age, condition, zip code of home, parental occupations, or hobbies that might result in exposing family members to lead, and if another child in the home has a history of elevated BLLs.
But a careful history for potential lead exposures can be time-consuming.
“There’s a lot to cover in a routine well child visit,” Dr. Buncher said. “We have maybe 15-20 minutes to cover a lot.”
Clinics also vary on whether lead screening questions are put into workflows in the electronic medical record. Although parents can complete a written questionnaire about possible lead exposures, they may have difficulty answering questions about the age of their home or not know whether their occupation is high risk.
Transportation to a clinic is often a barrier for families, and sometimes patients must travel to a separate lab to be tested for lead.
Dr. Buncher also pointed to the patchwork of local and state requirements that can lead to confusion among providers. Massachusetts, where he formerly practiced, has a universal requirement to test all children at ages 1, 2, and 3 years. But in Pennsylvania, screening laws vary from county to county.
“Pennsylvania should implement universal screening recommendations for all kids under 6 regardless of what county you live in,” Dr. Buncher said.
Protective Measures
Alan Woolf, MD, a professor of pediatrics at Harvard Medical School, Boston, Massachusetts, and director of the Pediatric Environmental Health Center at Boston Children’s Hospital, has a few ideas about how providers can step up their lead game, including partnering with their local health department.
The CDC funds Childhood Lead Poisoning Prevention Programs based in state and local health departments to work with clinicians to improve rates of blood lead testing, monitor the prevalence of lead in their jurisdictions, and ensure that a system of referral is available for treatment and lead remediation services in the home.
Dr. Woolf also suggested that clinicians refer patients under age 3 years with high BLLs to their local Early Intervention Program.
“They’ll assess their child’s development, their speech, their motor skills, their social skills, and if they qualify, it’s free,” Dr. Woolf said.
He cited research showing children with elevated lead levels who received early intervention services performed better in grade school than equally exposed children who did not access similar services.
Another key strategy for pediatric clinicians is to learn local or state regulations for testing children for lead and how to access lead surveillance data in their practice area. Children who reside in high-risk areas are automatic candidates for screening.
Dr. Woolf pointed out that big cities are not the only localities with lead in the drinking water. If families are drawing water from their own well, they should collect that water annually to have it tested for lead and microbes.
At the clinic-wide level, Dr. Woolf recommends the use of blood lead testing as a quality improvement measure. For example, Akron Children’s Hospital developed a quality improvement initiative using a clinical decision support tool to raise screening rates in their network of 30 clinics. One year after beginning the project, lead screenings during 12-month well visits increased from 71% to 96%.
“What we’re interested in as pediatric health professionals is eliminating all background sources of lead in a child’s environment,” Dr. Woolf said. “Whether that’s applesauce pouches, whether that’s lead-containing paint, lead in water, lead in spices, or lead in imported pottery or cookware — there are just a tremendous number of sources of lead that we can do something about.”
None of the subjects reported financial conflicts of interest.
A former pediatrician, Dr. Thomas is a freelance science writer living in Portland, Oregon.
A version of this article appeared on Medscape.com.
following a 2023 outbreak of elevated levels of lead in children associated with consumption of contaminated applesauce.
Federal legislation in the 1970s eliminated lead from gasoline, paints, and other consumer products, and resulted in significantly reduced blood lead levels (BLLs) in children throughout the United States.
But recently published studies highlight persistent issues with lead in drinking water and consumer products, suggesting that the fight is not over.
It’s in the Water
In 2014 the city of Flint, Michigan, changed its water supply and high levels of lead were later found in the municipal water supply.
Effects of that crisis still plague the city today. An initial study found that elevated BLLs had doubled among children between 2013 and 2015.
Lead exposure in young children is associated with several negative outcomes, including decreased cognitive ability, brain volume, and social mobility, and increased anxiety/depression and impulsivity, and higher rates of criminal offenses later in life.
Many other water systems still contain lead pipes, despite a 1986 ban by the US Environmental Protection Agency on using them for installing or repairing public water systems. The mayor of Chicago announced a plan to start replacing lead service lines in 2020; however, 400,000 households are still served by these pipes, the most in the nation.
Benjamin Huynh, a native of Chicago, was curious about the impact of all those lead service lines. Now an assistant professor in the Department of Environmental Health and Engineering at Johns Hopkins University in Baltimore, Maryland, he and his colleagues researched how many children under the age of 6 years were exposed to contaminated water.
The results showed that lead contamination of water is widespread.
“We’re estimating that 68% of kids under the age of 6 in Chicago were exposed to lead-contaminated drinking water,” Mr. Huynh said.
He added that residents in predominantly Black and Latino neighborhoods had the highest risk for lead contamination in their water, but children living on these blocks were less likely to get tested, suggesting a need for more outreach to raise awareness.
Meanwhile, a little over one third of Chicago residents reported drinking bottled water as their main source of drinking water.
But even bottled water could contain lead. The US Food and Drug Administration (FDA) has set a limit for lead in bottled water to five parts per billion. The FDA threshold for taking action in public drinking water systems is 15 parts per billion. But the American Academy of Pediatrics states that no amount of lead in drinking water is considered safe for drinking.
Mr. Huynh also pointed out that not all home water filters remove lead. Only devices that meet National Sanitation Foundation 53 standards are certified for lead removal. Consumers should verify that the filter package specifically lists the device as certified for removing contaminant lead.
Lead-tainted Cinnamon
Last fall, the North Carolina Department of Health and Human Services identified several children with elevated levels of lead who had consumed WanaBana Apple Cinnamon Fruit Puree pouches.
An investigation by the FDA identified additional brands containing lead and issued a recall of applesauce pouches sold by retailers like Dollar Tree and Amazon.
According to the US Centers for Disease Control and Prevention, nearly 500 children were affected by the tainted applesauce. The FDA traced the source of the lead to cinnamon from a supplier in Ecuador.
An FDA spokesperson told this news organization the episode appears to have resulted from “economically motivated adulteration,” which occurs when a manufacturer leaves out or substitutes a valuable ingredient or part of a food. In the case of spices, lead may be added as a coloring agent or to increase the product weight.
“When we look at domestically made products from large, reputable companies, in general, they do a pretty good job of following safe product guidelines and regulations,” said Kevin Osterhoudt, MD, professor of pediatrics at the Perelman School of Medicine at the University of Pennsylvania in Philadelphia. “But when we use third-party sellers and we import things from other countries that aren’t regulated as closely, we certainly take a lot more risk in the products that we receive.”
While the Food Safety Modernization Act of 2011 aimed to improve agency’s capacity to manage the ever-rising volume of food produced domestically and imported from overseas, the funding has stayed flat while the volume of inspections has increased. In the early 1990s, the number of shipments screened by the agency numbered in the thousands annually. Last year the FDA screened 15 million shipments from more than 200 countries, according to the agency.
Prompted by the finding of lead in applesauce, the FDA began a wider investigation into ground cinnamon by sampling the product from discount retail stores. It recalled an additional six brands of cinnamon sold in the United States containing lead.
Dr. Osterhoudt’s message to families who think their child might have been exposed to a contaminated product is to dispose of it as directed by FDA and CDC guidelines.
In Philadelphia, where Dr. Osterhoudt practices as an emergency room physician, baseline rates of childhood lead poisoning are already high, so he advises families to “do a larger inventory of all the source potential sources of lead in their life and to reduce all the exposures as low as possible.”
He also advises parents that a nutritious diet high in calcium and iron can protect their children from the deleterious effects of lead.
Current Standards for Lead Screening and Testing
Lead is ubiquitous. The common routes of exposure to humans include use of fossil fuels such as leaded gasoline, some types of industrial facilities, and past use of lead-based paint in homes. In addition to spices, lead has been found in a wide variety of products such as toys, jewelry, antiques, cosmetics, and dietary supplements imported from other countries.
Noah Buncher, DO, is a primary care pediatrician in South Philadelphia at Children’s Hospital of Pennsylvania and the former director of a lead clinic in Boston that provides care for children with lead poisoning. He follows guidelines from the American Academy of Pediatrics that define an elevated BLL as ≥ 3.5 µg/dL. The guidelines recommend screening children for lead exposures during well child visits starting at age 6 months up to 6 years and obtaining a BLL if risks for lead exposure are present.
Dr. Buncher starts with a basic environmental history that covers items like the age, condition, zip code of home, parental occupations, or hobbies that might result in exposing family members to lead, and if another child in the home has a history of elevated BLLs.
But a careful history for potential lead exposures can be time-consuming.
“There’s a lot to cover in a routine well child visit,” Dr. Buncher said. “We have maybe 15-20 minutes to cover a lot.”
Clinics also vary on whether lead screening questions are put into workflows in the electronic medical record. Although parents can complete a written questionnaire about possible lead exposures, they may have difficulty answering questions about the age of their home or not know whether their occupation is high risk.
Transportation to a clinic is often a barrier for families, and sometimes patients must travel to a separate lab to be tested for lead.
Dr. Buncher also pointed to the patchwork of local and state requirements that can lead to confusion among providers. Massachusetts, where he formerly practiced, has a universal requirement to test all children at ages 1, 2, and 3 years. But in Pennsylvania, screening laws vary from county to county.
“Pennsylvania should implement universal screening recommendations for all kids under 6 regardless of what county you live in,” Dr. Buncher said.
Protective Measures
Alan Woolf, MD, a professor of pediatrics at Harvard Medical School, Boston, Massachusetts, and director of the Pediatric Environmental Health Center at Boston Children’s Hospital, has a few ideas about how providers can step up their lead game, including partnering with their local health department.
The CDC funds Childhood Lead Poisoning Prevention Programs based in state and local health departments to work with clinicians to improve rates of blood lead testing, monitor the prevalence of lead in their jurisdictions, and ensure that a system of referral is available for treatment and lead remediation services in the home.
Dr. Woolf also suggested that clinicians refer patients under age 3 years with high BLLs to their local Early Intervention Program.
“They’ll assess their child’s development, their speech, their motor skills, their social skills, and if they qualify, it’s free,” Dr. Woolf said.
He cited research showing children with elevated lead levels who received early intervention services performed better in grade school than equally exposed children who did not access similar services.
Another key strategy for pediatric clinicians is to learn local or state regulations for testing children for lead and how to access lead surveillance data in their practice area. Children who reside in high-risk areas are automatic candidates for screening.
Dr. Woolf pointed out that big cities are not the only localities with lead in the drinking water. If families are drawing water from their own well, they should collect that water annually to have it tested for lead and microbes.
At the clinic-wide level, Dr. Woolf recommends the use of blood lead testing as a quality improvement measure. For example, Akron Children’s Hospital developed a quality improvement initiative using a clinical decision support tool to raise screening rates in their network of 30 clinics. One year after beginning the project, lead screenings during 12-month well visits increased from 71% to 96%.
“What we’re interested in as pediatric health professionals is eliminating all background sources of lead in a child’s environment,” Dr. Woolf said. “Whether that’s applesauce pouches, whether that’s lead-containing paint, lead in water, lead in spices, or lead in imported pottery or cookware — there are just a tremendous number of sources of lead that we can do something about.”
None of the subjects reported financial conflicts of interest.
A former pediatrician, Dr. Thomas is a freelance science writer living in Portland, Oregon.
A version of this article appeared on Medscape.com.
Oncology Practice and Lab to Pay $4 Million in Kickback Case
The US Department of Justice (DOJ) announced on April 2 that Oncology San Antonio, PA, and its physicians have agreed to pay $1.3 million, and CorePath Laboratories, PA, has agreed to pay nearly $2.75 million plus accrued interest in civil settlements with the United States and Texas for alleged violations of the False Claims Act.
According to the DOJ, the diagnostic reference laboratory, CorePath Laboratories, conducted in-office bone marrow biopsies at Oncology San Antonio practice locations and performed diagnostic testing on the samples. CorePath Laboratories agreed to pay $115 for each biopsy referred by Oncology San Antonio physicians, and these biopsy payments were allegedly paid to the private practices of three physicians at Oncology San Antonio. This arrangement allegedly began in August 2016.
The DOJ claimed that the payments for referring biopsies constituted illegal kickbacks under the Anti-Kickback Statute, which prohibits offering or receiving payments to encourage referrals of services covered by federal healthcare programs like Medicare and Medicaid.
“Violations of the Anti-Kickback Statute involving oncology services can waste scarce federal healthcare program funds and corrupt the medical decision-making process,” Special Agent in Charge Jason E. Meadows with the US Department of Health and Human Services Office of Inspector General said in a statement.
Oncology San Antonio told this news organization that the cost and distraction of prolonged litigation were the primary factors in its decision to settle. “The decision to settle was an extremely difficult one because Oncology San Antonio was confident that it would have prevailed in any action,” the practice said via email.
This civil settlement with Oncology San Antonio also resolved allegations that a physician affiliated with the practice, Jayasree Rao, MD, provided unnecessary tests, services, and treatments to patients covered by Medicare, TRICARE, and Texas Medicaid in the San Antonio Metro Area and billed these federal healthcare programs for the unnecessary services.
The DOJ identified Slavisa Gasic, MD, a physician formerly employed by Dr. Rao, as a whistleblower in the investigation. When asked for comment, Oncology San Antonio alleged Dr. Gasic was “disgruntled for not being promoted.”
According to Oncology San Antonio, the contract for bone marrow biopsies was negotiated and signed by a former nonphysician officer of the company without the input of Oncology San Antonio physicians. The contract permitted bone marrow biopsies at Oncology San Antonio clinics instead of requiring older adult and sick patients to go to a different facility for these services.
“Oncology San Antonio and Rao vehemently denied Gasic’s allegations as wholly unfounded,” the company told this news organization.
Dr. Rao retired in March and is no longer practicing. CorePath Laboratories, PA, did not respond to this news organization’s request for comment.
According to the DOJ press release, the “investigation and resolution of this matter illustrate the government’s emphasis on combating healthcare fraud.”
A version of this article appeared on Medscape.com.
The US Department of Justice (DOJ) announced on April 2 that Oncology San Antonio, PA, and its physicians have agreed to pay $1.3 million, and CorePath Laboratories, PA, has agreed to pay nearly $2.75 million plus accrued interest in civil settlements with the United States and Texas for alleged violations of the False Claims Act.
According to the DOJ, the diagnostic reference laboratory, CorePath Laboratories, conducted in-office bone marrow biopsies at Oncology San Antonio practice locations and performed diagnostic testing on the samples. CorePath Laboratories agreed to pay $115 for each biopsy referred by Oncology San Antonio physicians, and these biopsy payments were allegedly paid to the private practices of three physicians at Oncology San Antonio. This arrangement allegedly began in August 2016.
The DOJ claimed that the payments for referring biopsies constituted illegal kickbacks under the Anti-Kickback Statute, which prohibits offering or receiving payments to encourage referrals of services covered by federal healthcare programs like Medicare and Medicaid.
“Violations of the Anti-Kickback Statute involving oncology services can waste scarce federal healthcare program funds and corrupt the medical decision-making process,” Special Agent in Charge Jason E. Meadows with the US Department of Health and Human Services Office of Inspector General said in a statement.
Oncology San Antonio told this news organization that the cost and distraction of prolonged litigation were the primary factors in its decision to settle. “The decision to settle was an extremely difficult one because Oncology San Antonio was confident that it would have prevailed in any action,” the practice said via email.
This civil settlement with Oncology San Antonio also resolved allegations that a physician affiliated with the practice, Jayasree Rao, MD, provided unnecessary tests, services, and treatments to patients covered by Medicare, TRICARE, and Texas Medicaid in the San Antonio Metro Area and billed these federal healthcare programs for the unnecessary services.
The DOJ identified Slavisa Gasic, MD, a physician formerly employed by Dr. Rao, as a whistleblower in the investigation. When asked for comment, Oncology San Antonio alleged Dr. Gasic was “disgruntled for not being promoted.”
According to Oncology San Antonio, the contract for bone marrow biopsies was negotiated and signed by a former nonphysician officer of the company without the input of Oncology San Antonio physicians. The contract permitted bone marrow biopsies at Oncology San Antonio clinics instead of requiring older adult and sick patients to go to a different facility for these services.
“Oncology San Antonio and Rao vehemently denied Gasic’s allegations as wholly unfounded,” the company told this news organization.
Dr. Rao retired in March and is no longer practicing. CorePath Laboratories, PA, did not respond to this news organization’s request for comment.
According to the DOJ press release, the “investigation and resolution of this matter illustrate the government’s emphasis on combating healthcare fraud.”
A version of this article appeared on Medscape.com.
The US Department of Justice (DOJ) announced on April 2 that Oncology San Antonio, PA, and its physicians have agreed to pay $1.3 million, and CorePath Laboratories, PA, has agreed to pay nearly $2.75 million plus accrued interest in civil settlements with the United States and Texas for alleged violations of the False Claims Act.
According to the DOJ, the diagnostic reference laboratory, CorePath Laboratories, conducted in-office bone marrow biopsies at Oncology San Antonio practice locations and performed diagnostic testing on the samples. CorePath Laboratories agreed to pay $115 for each biopsy referred by Oncology San Antonio physicians, and these biopsy payments were allegedly paid to the private practices of three physicians at Oncology San Antonio. This arrangement allegedly began in August 2016.
The DOJ claimed that the payments for referring biopsies constituted illegal kickbacks under the Anti-Kickback Statute, which prohibits offering or receiving payments to encourage referrals of services covered by federal healthcare programs like Medicare and Medicaid.
“Violations of the Anti-Kickback Statute involving oncology services can waste scarce federal healthcare program funds and corrupt the medical decision-making process,” Special Agent in Charge Jason E. Meadows with the US Department of Health and Human Services Office of Inspector General said in a statement.
Oncology San Antonio told this news organization that the cost and distraction of prolonged litigation were the primary factors in its decision to settle. “The decision to settle was an extremely difficult one because Oncology San Antonio was confident that it would have prevailed in any action,” the practice said via email.
This civil settlement with Oncology San Antonio also resolved allegations that a physician affiliated with the practice, Jayasree Rao, MD, provided unnecessary tests, services, and treatments to patients covered by Medicare, TRICARE, and Texas Medicaid in the San Antonio Metro Area and billed these federal healthcare programs for the unnecessary services.
The DOJ identified Slavisa Gasic, MD, a physician formerly employed by Dr. Rao, as a whistleblower in the investigation. When asked for comment, Oncology San Antonio alleged Dr. Gasic was “disgruntled for not being promoted.”
According to Oncology San Antonio, the contract for bone marrow biopsies was negotiated and signed by a former nonphysician officer of the company without the input of Oncology San Antonio physicians. The contract permitted bone marrow biopsies at Oncology San Antonio clinics instead of requiring older adult and sick patients to go to a different facility for these services.
“Oncology San Antonio and Rao vehemently denied Gasic’s allegations as wholly unfounded,” the company told this news organization.
Dr. Rao retired in March and is no longer practicing. CorePath Laboratories, PA, did not respond to this news organization’s request for comment.
According to the DOJ press release, the “investigation and resolution of this matter illustrate the government’s emphasis on combating healthcare fraud.”
A version of this article appeared on Medscape.com.
Botanical Briefs: Fig Phytophotodermatitis (Ficus carica)
Plant Parts and Nomenclature
Ficus carica (common fig) is a deciduous shrub or small tree with smooth gray bark that can grow up to 10 m in height (Figure 1). It is characterized by many spreading branches, but the trunk rarely grows beyond a diameter of 7 in. Its hairy leaves are coarse on the upper side and soft underneath with 3 to 7 deep lobes that can extend up to 25 cm in length or width; the leaves grow individually, alternating along the sides of the branches. Fig trees often can be seen adorning yards, gardens, and parks, especially in tropical and subtropical climates. Ficus carica should not be confused with Ficus benjamina (weeping fig), a common ornamental tree that also is used to provide shade in hot climates, though both can cause phototoxic skin eruptions.

The common fig tree originated in the Mediterranean and western Asia1 and has been cultivated by humans since the second and third millennia

Ficus carica is a member of the Moraceae family (derived from the Latin name for the mulberry tree), which includes 53 genera and approximately 1400 species, of which about 850 belong to the genus Ficus (the Latin name for a fig tree). The term carica likely comes from the Latin word carricare (to load) to describe a tree loaded with figs. Family members include trees, shrubs, lianas, and herbs that usually contain laticifers with a milky latex.
Traditional Uses
For centuries, components of the fig tree have been used in herbal teas and pastes to treat ailments ranging from sore throats to diarrhea, though there is no evidence to support their efficacy.4 Ancient Indians and Egyptians used plants such as the common fig tree containing furocoumarins to induce hyperpigmentation in vitiligo.5
Phototoxic Components
The leaves and sap of the common fig tree contain psoralens, which are members of the furocoumarin group of chemical compounds and are the source of its phototoxicity. The fruit does not contain psoralens.6-9 The tree also produces proteolytic enzymes such as protease, amylase, ficin, triterpenoids, and lipodiastase that enhance its phototoxic effects.8 Exposure to UV light between 320 and 400 nm following contact with these phototoxic components triggers a reaction in the skin over the course of 1 to 3 days.5 The psoralens bind in epidermal cells, cross-link the DNA, and cause cell-membrane destruction, leading to edema and necrosis.10 The delay in symptoms may be attributed to the time needed to synthesize acute-phase reaction proteins such as tumor necrosis factor α and IL-1.11 In spring and summer months, an increased concentration of psoralens in the leaves and sap contribute to an increased incidence of phytophotodermatitis.9 Humidity and sweat also increase the percutaneous absorption of psoralens.12,13
Allergens
Fig trees produce a latex protein that can cause cross-reactive hypersensitivity reactions in those allergic to F benjamina latex and rubber latex.6 The latex proteins in fig trees can act as airborne respiratory allergens. Ingestion of figs can produce anaphylactic reactions in those sensitized to rubber latex and F benjamina latex.7 Other plant families associated with phototoxic reactions include Rutaceae (lemon, lime, bitter orange), Apiaceae (formerly Umbelliferae)(carrot, parsnip, parsley, dill, celery, hogweed), and Fabaceae (prairie turnip).
Cutaneous Manifestations
Most cases of fig phytophotodermatitis begin with burning, pain, and/or itching within hours of sunlight exposure in areas of the skin that encountered components of the fig tree, often in a linear pattern. The affected areas become erythematous and edematous with formation of bullae and unilocular vesicles over the course of 1 to 3 days.12,14,15 Lesions may extend beyond the region of contact with the fig tree as they spread across the skin due to sweat or friction, and pain may linger even after the lesions resolve.12,13,16 Adults who handle fig trees (eg, pruning) are susceptible to phototoxic reactions, especially those using chain saws or other mechanisms that result in spray exposure, as the photosensitizing sap permeates the wood and bark of the entire tree.17 Similarly, children who handle fig leaves or sap during outdoor play can develop bullous eruptions. Severe cases have resulted in hospital admission after prolonged exposure.16 Additionally, irritant dermatitis may arise from contact with the trichomes or “hairs” on various parts of the plant.

Patients who use natural remedies containing components of the fig tree without the supervision of a medical provider put themselves at risk for unsafe or unwanted adverse effects, such as phytophotodermatitis.12,15,16,18 An entire family presented with burns after they applied fig leaf extract to the skin prior to tanning outside in the sun.19 A 42-year-old woman acquired a severe burn covering 81% of the body surface after topically applying fig leaf tea to the skin as a tanning agent.20 A subset of patients ingesting or applying fig tree components for conditions such as vitiligo, dermatitis, onychomycosis, and motor retardation developed similar cutaneous reactions.13,14,21,22 Lesions resembling finger marks can raise concerns for potential abuse or neglect in children.22
The differential diagnosis for fig phytophotodermatitis includes sunburn, chemical burns, drug-related photosensitivity, infectious lesions (eg, herpes simplex, bullous impetigo, Lyme disease, superficial lymphangitis), connective tissue disease (eg, systemic lupus erythematosus), contact dermatitis, and nonaccidental trauma.12,15,18 Compared to sunburn, phytophotodermatitis tends to increase in severity over days following exposure and heals with dramatic hyperpigmentation, which also prompts visits to dermatology.12
Treatment
Treatment of fig phytophotodermatitis chiefly is symptomatic, including analgesia, appropriate wound care, and infection prophylaxis. Topical and systemic corticosteroids may aid in the resolution of moderate to severe reactions.15,23,24 Even severe injuries over small areas or mild injuries to a high percentage of the total body surface area may require treatment in a burn unit. Patients should be encouraged to use mineral-based sunscreens on the affected areas to reduce the risk for hyperpigmentation. Individuals who regularly handle fig trees should use contact barriers including gloves and protective clothing (eg, long-sleeved shirts, long pants).
- Ikegami H, Nogata H, Hirashima K, et al. Analysis of genetic diversity among European and Asian fig varieties (Ficus carica L.) using ISSR, RAPD, and SSR markers. Genetic Resources and Crop Evolution. 2009;56:201-209.
- Zohary D, Spiegel-Roy P. Beginnings of fruit growing in the Old World. Science. 1975;187:319-327.
- Young R. Young’s Analytical Concordance. Thomas Nelson; 1982.
- Duke JA. Handbook of Medicinal Herbs. CRC Press; 2002.
- Pathak MA, Fitzpatrick TB. Bioassay of natural and synthetic furocoumarins (psoralens). J Invest Dermatol. 1959;32:509-518.
- Focke M, Hemmer W, Wöhrl S, et al. Cross-reactivity between Ficus benjamina latex and fig fruit in patients with clinical fig allergy. Clin Exp Allergy. 2003;33:971-977.
- Hemmer W, Focke M, Götz M, et al. Sensitization to Ficus benjamina: relationship to natural rubber latex allergy and identification of foods implicated in the Ficus-fruit syndrome. Clin Exp Allergy. 2004;34:1251-1258.
- Bonamonte D, Foti C, Lionetti N, et al. Photoallergic contact dermatitis to 8-methoxypsoralen in Ficus carica. Contact Dermatitis. 2010;62:343-348.
- Zaynoun ST, Aftimos BG, Abi Ali L, et al. Ficus carica; isolation and quantification of the photoactive components. Contact Dermatitis. 1984;11:21-25.
- Tessman JW, Isaacs ST, Hearst JE. Photochemistry of the furan-side 8-methoxypsoralen-thymidine monoadduct inside the DNA helix. conversion to diadduct and to pyrone-side monoadduct. Biochemistry. 1985;24:1669-1676.
- Geary P. Burns related to the use of psoralens as a tanning agent. Burns. 1996;22:636-637.
- Redgrave N, Solomon J. Severe phytophotodermatitis from fig sap: a little known phenomenon. BMJ Case Rep. 2021;14:E238745.
- Ozdamar E, Ozbek S, Akin S. An unusual cause of burn injury: fig leaf decoction used as a remedy for a dermatitis of unknown etiology. J Burn Care Rehabil. 2003;24:229-233; discussion 228.
- Berakha GJ, Lefkovits G. Psoralen phototherapy and phototoxicity. Ann Plast Surg. 1985;14:458-461.
- Papazoglou A, Mantadakis E. Fig tree leaves phytophotodermatitis. J Pediatr. 2021;239:244-245.
- Imen MS, Ahmadabadi A, Tavousi SH, et al. The curious cases of burn by fig tree leaves. Indian J Dermatol. 2019;64:71-73.
- Rouaiguia-Bouakkaz S, Amira-Guebailia H, Rivière C, et al. Identification and quantification of furanocoumarins in stem bark and wood of eight Algerian varieties of Ficus carica by RP-HPLC-DAD and RP-HPLC-DAD-MS. Nat Prod Commun. 2013;8:485-486.
- Oliveira AA, Morais J, Pires O, et al. Fig tree induced phytophotodermatitis. BMJ Case Rep. 2020;13:E233392.
- Bassioukas K, Stergiopoulou C, Hatzis J. Erythrodermic phytophotodermatitis after application of aqueous fig-leaf extract as an artificial suntan promoter and sunbathing. Contact Dermatitis. 2004;51:94-95.
- Sforza M, Andjelkov K, Zaccheddu R. Severe burn on 81% of body surface after sun tanning. Ulus Travma Acil Cerrahi Derg. 2013;19:383-384.
- Son JH, Jin H, You HS, et al. Five cases of phytophotodermatitis caused by fig leaves and relevant literature review. Ann Dermatol. 2017;29:86-90.
- Abali AE, Aka M, Aydogan C, et al. Burns or phytophotodermatitis, abuse or neglect: confusing aspects of skin lesions caused by the superstitious use of fig leaves. J Burn Care Res. 2012;33:E309-E312.
- Picard C, Morice C, Moreau A, et al. Phytophotodermatitis in children: a difficult diagnosis mimicking other dermatitis. 2017;5:1-3.
- Enjolras O, Soupre V, Picard A. Uncommon benign infantile vascular tumors. Adv Dermatol. 2008;24:105-124.
Plant Parts and Nomenclature
Ficus carica (common fig) is a deciduous shrub or small tree with smooth gray bark that can grow up to 10 m in height (Figure 1). It is characterized by many spreading branches, but the trunk rarely grows beyond a diameter of 7 in. Its hairy leaves are coarse on the upper side and soft underneath with 3 to 7 deep lobes that can extend up to 25 cm in length or width; the leaves grow individually, alternating along the sides of the branches. Fig trees often can be seen adorning yards, gardens, and parks, especially in tropical and subtropical climates. Ficus carica should not be confused with Ficus benjamina (weeping fig), a common ornamental tree that also is used to provide shade in hot climates, though both can cause phototoxic skin eruptions.

The common fig tree originated in the Mediterranean and western Asia1 and has been cultivated by humans since the second and third millennia

Ficus carica is a member of the Moraceae family (derived from the Latin name for the mulberry tree), which includes 53 genera and approximately 1400 species, of which about 850 belong to the genus Ficus (the Latin name for a fig tree). The term carica likely comes from the Latin word carricare (to load) to describe a tree loaded with figs. Family members include trees, shrubs, lianas, and herbs that usually contain laticifers with a milky latex.
Traditional Uses
For centuries, components of the fig tree have been used in herbal teas and pastes to treat ailments ranging from sore throats to diarrhea, though there is no evidence to support their efficacy.4 Ancient Indians and Egyptians used plants such as the common fig tree containing furocoumarins to induce hyperpigmentation in vitiligo.5
Phototoxic Components
The leaves and sap of the common fig tree contain psoralens, which are members of the furocoumarin group of chemical compounds and are the source of its phototoxicity. The fruit does not contain psoralens.6-9 The tree also produces proteolytic enzymes such as protease, amylase, ficin, triterpenoids, and lipodiastase that enhance its phototoxic effects.8 Exposure to UV light between 320 and 400 nm following contact with these phototoxic components triggers a reaction in the skin over the course of 1 to 3 days.5 The psoralens bind in epidermal cells, cross-link the DNA, and cause cell-membrane destruction, leading to edema and necrosis.10 The delay in symptoms may be attributed to the time needed to synthesize acute-phase reaction proteins such as tumor necrosis factor α and IL-1.11 In spring and summer months, an increased concentration of psoralens in the leaves and sap contribute to an increased incidence of phytophotodermatitis.9 Humidity and sweat also increase the percutaneous absorption of psoralens.12,13
Allergens
Fig trees produce a latex protein that can cause cross-reactive hypersensitivity reactions in those allergic to F benjamina latex and rubber latex.6 The latex proteins in fig trees can act as airborne respiratory allergens. Ingestion of figs can produce anaphylactic reactions in those sensitized to rubber latex and F benjamina latex.7 Other plant families associated with phototoxic reactions include Rutaceae (lemon, lime, bitter orange), Apiaceae (formerly Umbelliferae)(carrot, parsnip, parsley, dill, celery, hogweed), and Fabaceae (prairie turnip).
Cutaneous Manifestations
Most cases of fig phytophotodermatitis begin with burning, pain, and/or itching within hours of sunlight exposure in areas of the skin that encountered components of the fig tree, often in a linear pattern. The affected areas become erythematous and edematous with formation of bullae and unilocular vesicles over the course of 1 to 3 days.12,14,15 Lesions may extend beyond the region of contact with the fig tree as they spread across the skin due to sweat or friction, and pain may linger even after the lesions resolve.12,13,16 Adults who handle fig trees (eg, pruning) are susceptible to phototoxic reactions, especially those using chain saws or other mechanisms that result in spray exposure, as the photosensitizing sap permeates the wood and bark of the entire tree.17 Similarly, children who handle fig leaves or sap during outdoor play can develop bullous eruptions. Severe cases have resulted in hospital admission after prolonged exposure.16 Additionally, irritant dermatitis may arise from contact with the trichomes or “hairs” on various parts of the plant.

Patients who use natural remedies containing components of the fig tree without the supervision of a medical provider put themselves at risk for unsafe or unwanted adverse effects, such as phytophotodermatitis.12,15,16,18 An entire family presented with burns after they applied fig leaf extract to the skin prior to tanning outside in the sun.19 A 42-year-old woman acquired a severe burn covering 81% of the body surface after topically applying fig leaf tea to the skin as a tanning agent.20 A subset of patients ingesting or applying fig tree components for conditions such as vitiligo, dermatitis, onychomycosis, and motor retardation developed similar cutaneous reactions.13,14,21,22 Lesions resembling finger marks can raise concerns for potential abuse or neglect in children.22
The differential diagnosis for fig phytophotodermatitis includes sunburn, chemical burns, drug-related photosensitivity, infectious lesions (eg, herpes simplex, bullous impetigo, Lyme disease, superficial lymphangitis), connective tissue disease (eg, systemic lupus erythematosus), contact dermatitis, and nonaccidental trauma.12,15,18 Compared to sunburn, phytophotodermatitis tends to increase in severity over days following exposure and heals with dramatic hyperpigmentation, which also prompts visits to dermatology.12
Treatment
Treatment of fig phytophotodermatitis chiefly is symptomatic, including analgesia, appropriate wound care, and infection prophylaxis. Topical and systemic corticosteroids may aid in the resolution of moderate to severe reactions.15,23,24 Even severe injuries over small areas or mild injuries to a high percentage of the total body surface area may require treatment in a burn unit. Patients should be encouraged to use mineral-based sunscreens on the affected areas to reduce the risk for hyperpigmentation. Individuals who regularly handle fig trees should use contact barriers including gloves and protective clothing (eg, long-sleeved shirts, long pants).
Plant Parts and Nomenclature
Ficus carica (common fig) is a deciduous shrub or small tree with smooth gray bark that can grow up to 10 m in height (Figure 1). It is characterized by many spreading branches, but the trunk rarely grows beyond a diameter of 7 in. Its hairy leaves are coarse on the upper side and soft underneath with 3 to 7 deep lobes that can extend up to 25 cm in length or width; the leaves grow individually, alternating along the sides of the branches. Fig trees often can be seen adorning yards, gardens, and parks, especially in tropical and subtropical climates. Ficus carica should not be confused with Ficus benjamina (weeping fig), a common ornamental tree that also is used to provide shade in hot climates, though both can cause phototoxic skin eruptions.

The common fig tree originated in the Mediterranean and western Asia1 and has been cultivated by humans since the second and third millennia

Ficus carica is a member of the Moraceae family (derived from the Latin name for the mulberry tree), which includes 53 genera and approximately 1400 species, of which about 850 belong to the genus Ficus (the Latin name for a fig tree). The term carica likely comes from the Latin word carricare (to load) to describe a tree loaded with figs. Family members include trees, shrubs, lianas, and herbs that usually contain laticifers with a milky latex.
Traditional Uses
For centuries, components of the fig tree have been used in herbal teas and pastes to treat ailments ranging from sore throats to diarrhea, though there is no evidence to support their efficacy.4 Ancient Indians and Egyptians used plants such as the common fig tree containing furocoumarins to induce hyperpigmentation in vitiligo.5
Phototoxic Components
The leaves and sap of the common fig tree contain psoralens, which are members of the furocoumarin group of chemical compounds and are the source of its phototoxicity. The fruit does not contain psoralens.6-9 The tree also produces proteolytic enzymes such as protease, amylase, ficin, triterpenoids, and lipodiastase that enhance its phototoxic effects.8 Exposure to UV light between 320 and 400 nm following contact with these phototoxic components triggers a reaction in the skin over the course of 1 to 3 days.5 The psoralens bind in epidermal cells, cross-link the DNA, and cause cell-membrane destruction, leading to edema and necrosis.10 The delay in symptoms may be attributed to the time needed to synthesize acute-phase reaction proteins such as tumor necrosis factor α and IL-1.11 In spring and summer months, an increased concentration of psoralens in the leaves and sap contribute to an increased incidence of phytophotodermatitis.9 Humidity and sweat also increase the percutaneous absorption of psoralens.12,13
Allergens
Fig trees produce a latex protein that can cause cross-reactive hypersensitivity reactions in those allergic to F benjamina latex and rubber latex.6 The latex proteins in fig trees can act as airborne respiratory allergens. Ingestion of figs can produce anaphylactic reactions in those sensitized to rubber latex and F benjamina latex.7 Other plant families associated with phototoxic reactions include Rutaceae (lemon, lime, bitter orange), Apiaceae (formerly Umbelliferae)(carrot, parsnip, parsley, dill, celery, hogweed), and Fabaceae (prairie turnip).
Cutaneous Manifestations
Most cases of fig phytophotodermatitis begin with burning, pain, and/or itching within hours of sunlight exposure in areas of the skin that encountered components of the fig tree, often in a linear pattern. The affected areas become erythematous and edematous with formation of bullae and unilocular vesicles over the course of 1 to 3 days.12,14,15 Lesions may extend beyond the region of contact with the fig tree as they spread across the skin due to sweat or friction, and pain may linger even after the lesions resolve.12,13,16 Adults who handle fig trees (eg, pruning) are susceptible to phototoxic reactions, especially those using chain saws or other mechanisms that result in spray exposure, as the photosensitizing sap permeates the wood and bark of the entire tree.17 Similarly, children who handle fig leaves or sap during outdoor play can develop bullous eruptions. Severe cases have resulted in hospital admission after prolonged exposure.16 Additionally, irritant dermatitis may arise from contact with the trichomes or “hairs” on various parts of the plant.

Patients who use natural remedies containing components of the fig tree without the supervision of a medical provider put themselves at risk for unsafe or unwanted adverse effects, such as phytophotodermatitis.12,15,16,18 An entire family presented with burns after they applied fig leaf extract to the skin prior to tanning outside in the sun.19 A 42-year-old woman acquired a severe burn covering 81% of the body surface after topically applying fig leaf tea to the skin as a tanning agent.20 A subset of patients ingesting or applying fig tree components for conditions such as vitiligo, dermatitis, onychomycosis, and motor retardation developed similar cutaneous reactions.13,14,21,22 Lesions resembling finger marks can raise concerns for potential abuse or neglect in children.22
The differential diagnosis for fig phytophotodermatitis includes sunburn, chemical burns, drug-related photosensitivity, infectious lesions (eg, herpes simplex, bullous impetigo, Lyme disease, superficial lymphangitis), connective tissue disease (eg, systemic lupus erythematosus), contact dermatitis, and nonaccidental trauma.12,15,18 Compared to sunburn, phytophotodermatitis tends to increase in severity over days following exposure and heals with dramatic hyperpigmentation, which also prompts visits to dermatology.12
Treatment
Treatment of fig phytophotodermatitis chiefly is symptomatic, including analgesia, appropriate wound care, and infection prophylaxis. Topical and systemic corticosteroids may aid in the resolution of moderate to severe reactions.15,23,24 Even severe injuries over small areas or mild injuries to a high percentage of the total body surface area may require treatment in a burn unit. Patients should be encouraged to use mineral-based sunscreens on the affected areas to reduce the risk for hyperpigmentation. Individuals who regularly handle fig trees should use contact barriers including gloves and protective clothing (eg, long-sleeved shirts, long pants).
- Ikegami H, Nogata H, Hirashima K, et al. Analysis of genetic diversity among European and Asian fig varieties (Ficus carica L.) using ISSR, RAPD, and SSR markers. Genetic Resources and Crop Evolution. 2009;56:201-209.
- Zohary D, Spiegel-Roy P. Beginnings of fruit growing in the Old World. Science. 1975;187:319-327.
- Young R. Young’s Analytical Concordance. Thomas Nelson; 1982.
- Duke JA. Handbook of Medicinal Herbs. CRC Press; 2002.
- Pathak MA, Fitzpatrick TB. Bioassay of natural and synthetic furocoumarins (psoralens). J Invest Dermatol. 1959;32:509-518.
- Focke M, Hemmer W, Wöhrl S, et al. Cross-reactivity between Ficus benjamina latex and fig fruit in patients with clinical fig allergy. Clin Exp Allergy. 2003;33:971-977.
- Hemmer W, Focke M, Götz M, et al. Sensitization to Ficus benjamina: relationship to natural rubber latex allergy and identification of foods implicated in the Ficus-fruit syndrome. Clin Exp Allergy. 2004;34:1251-1258.
- Bonamonte D, Foti C, Lionetti N, et al. Photoallergic contact dermatitis to 8-methoxypsoralen in Ficus carica. Contact Dermatitis. 2010;62:343-348.
- Zaynoun ST, Aftimos BG, Abi Ali L, et al. Ficus carica; isolation and quantification of the photoactive components. Contact Dermatitis. 1984;11:21-25.
- Tessman JW, Isaacs ST, Hearst JE. Photochemistry of the furan-side 8-methoxypsoralen-thymidine monoadduct inside the DNA helix. conversion to diadduct and to pyrone-side monoadduct. Biochemistry. 1985;24:1669-1676.
- Geary P. Burns related to the use of psoralens as a tanning agent. Burns. 1996;22:636-637.
- Redgrave N, Solomon J. Severe phytophotodermatitis from fig sap: a little known phenomenon. BMJ Case Rep. 2021;14:E238745.
- Ozdamar E, Ozbek S, Akin S. An unusual cause of burn injury: fig leaf decoction used as a remedy for a dermatitis of unknown etiology. J Burn Care Rehabil. 2003;24:229-233; discussion 228.
- Berakha GJ, Lefkovits G. Psoralen phototherapy and phototoxicity. Ann Plast Surg. 1985;14:458-461.
- Papazoglou A, Mantadakis E. Fig tree leaves phytophotodermatitis. J Pediatr. 2021;239:244-245.
- Imen MS, Ahmadabadi A, Tavousi SH, et al. The curious cases of burn by fig tree leaves. Indian J Dermatol. 2019;64:71-73.
- Rouaiguia-Bouakkaz S, Amira-Guebailia H, Rivière C, et al. Identification and quantification of furanocoumarins in stem bark and wood of eight Algerian varieties of Ficus carica by RP-HPLC-DAD and RP-HPLC-DAD-MS. Nat Prod Commun. 2013;8:485-486.
- Oliveira AA, Morais J, Pires O, et al. Fig tree induced phytophotodermatitis. BMJ Case Rep. 2020;13:E233392.
- Bassioukas K, Stergiopoulou C, Hatzis J. Erythrodermic phytophotodermatitis after application of aqueous fig-leaf extract as an artificial suntan promoter and sunbathing. Contact Dermatitis. 2004;51:94-95.
- Sforza M, Andjelkov K, Zaccheddu R. Severe burn on 81% of body surface after sun tanning. Ulus Travma Acil Cerrahi Derg. 2013;19:383-384.
- Son JH, Jin H, You HS, et al. Five cases of phytophotodermatitis caused by fig leaves and relevant literature review. Ann Dermatol. 2017;29:86-90.
- Abali AE, Aka M, Aydogan C, et al. Burns or phytophotodermatitis, abuse or neglect: confusing aspects of skin lesions caused by the superstitious use of fig leaves. J Burn Care Res. 2012;33:E309-E312.
- Picard C, Morice C, Moreau A, et al. Phytophotodermatitis in children: a difficult diagnosis mimicking other dermatitis. 2017;5:1-3.
- Enjolras O, Soupre V, Picard A. Uncommon benign infantile vascular tumors. Adv Dermatol. 2008;24:105-124.
- Ikegami H, Nogata H, Hirashima K, et al. Analysis of genetic diversity among European and Asian fig varieties (Ficus carica L.) using ISSR, RAPD, and SSR markers. Genetic Resources and Crop Evolution. 2009;56:201-209.
- Zohary D, Spiegel-Roy P. Beginnings of fruit growing in the Old World. Science. 1975;187:319-327.
- Young R. Young’s Analytical Concordance. Thomas Nelson; 1982.
- Duke JA. Handbook of Medicinal Herbs. CRC Press; 2002.
- Pathak MA, Fitzpatrick TB. Bioassay of natural and synthetic furocoumarins (psoralens). J Invest Dermatol. 1959;32:509-518.
- Focke M, Hemmer W, Wöhrl S, et al. Cross-reactivity between Ficus benjamina latex and fig fruit in patients with clinical fig allergy. Clin Exp Allergy. 2003;33:971-977.
- Hemmer W, Focke M, Götz M, et al. Sensitization to Ficus benjamina: relationship to natural rubber latex allergy and identification of foods implicated in the Ficus-fruit syndrome. Clin Exp Allergy. 2004;34:1251-1258.
- Bonamonte D, Foti C, Lionetti N, et al. Photoallergic contact dermatitis to 8-methoxypsoralen in Ficus carica. Contact Dermatitis. 2010;62:343-348.
- Zaynoun ST, Aftimos BG, Abi Ali L, et al. Ficus carica; isolation and quantification of the photoactive components. Contact Dermatitis. 1984;11:21-25.
- Tessman JW, Isaacs ST, Hearst JE. Photochemistry of the furan-side 8-methoxypsoralen-thymidine monoadduct inside the DNA helix. conversion to diadduct and to pyrone-side monoadduct. Biochemistry. 1985;24:1669-1676.
- Geary P. Burns related to the use of psoralens as a tanning agent. Burns. 1996;22:636-637.
- Redgrave N, Solomon J. Severe phytophotodermatitis from fig sap: a little known phenomenon. BMJ Case Rep. 2021;14:E238745.
- Ozdamar E, Ozbek S, Akin S. An unusual cause of burn injury: fig leaf decoction used as a remedy for a dermatitis of unknown etiology. J Burn Care Rehabil. 2003;24:229-233; discussion 228.
- Berakha GJ, Lefkovits G. Psoralen phototherapy and phototoxicity. Ann Plast Surg. 1985;14:458-461.
- Papazoglou A, Mantadakis E. Fig tree leaves phytophotodermatitis. J Pediatr. 2021;239:244-245.
- Imen MS, Ahmadabadi A, Tavousi SH, et al. The curious cases of burn by fig tree leaves. Indian J Dermatol. 2019;64:71-73.
- Rouaiguia-Bouakkaz S, Amira-Guebailia H, Rivière C, et al. Identification and quantification of furanocoumarins in stem bark and wood of eight Algerian varieties of Ficus carica by RP-HPLC-DAD and RP-HPLC-DAD-MS. Nat Prod Commun. 2013;8:485-486.
- Oliveira AA, Morais J, Pires O, et al. Fig tree induced phytophotodermatitis. BMJ Case Rep. 2020;13:E233392.
- Bassioukas K, Stergiopoulou C, Hatzis J. Erythrodermic phytophotodermatitis after application of aqueous fig-leaf extract as an artificial suntan promoter and sunbathing. Contact Dermatitis. 2004;51:94-95.
- Sforza M, Andjelkov K, Zaccheddu R. Severe burn on 81% of body surface after sun tanning. Ulus Travma Acil Cerrahi Derg. 2013;19:383-384.
- Son JH, Jin H, You HS, et al. Five cases of phytophotodermatitis caused by fig leaves and relevant literature review. Ann Dermatol. 2017;29:86-90.
- Abali AE, Aka M, Aydogan C, et al. Burns or phytophotodermatitis, abuse or neglect: confusing aspects of skin lesions caused by the superstitious use of fig leaves. J Burn Care Res. 2012;33:E309-E312.
- Picard C, Morice C, Moreau A, et al. Phytophotodermatitis in children: a difficult diagnosis mimicking other dermatitis. 2017;5:1-3.
- Enjolras O, Soupre V, Picard A. Uncommon benign infantile vascular tumors. Adv Dermatol. 2008;24:105-124.
Practice Points
- Exposure to the components of the common fig tree (Ficus carica) can induce phytophotodermatitis.
- Notable postinflammatory hyperpigmentation typically occurs in the healing stage of fig phytophotodermatitis.