User login
Ultrasound and Its Role In Diagnosing and Managing Endometriosis
Introduction: Imaging for Endometriosis — A Necessary Prerequisite
While the gold standard in the diagnosis of endometriosis remains laparoscopy, it is now recognized that thorough evaluation via ultrasound offers an acceptable, less expensive, and less invasive alternative. It is especially useful for the diagnosis of deep infiltrative disease, which penetrates more than 5 mm into the peritoneum, ovarian endometrioma, and when anatomic distortion occurs, such as to the path of the ureter.
Besides establishing the diagnosis, ultrasound imaging has become, along with MRI, the most important aid for proper preoperative planning. Not only does imaging provide the surgeon and patient with knowledge regarding the extent of the upcoming procedure, but it also allows the minimally invasive gynecologic (MIG) surgeon to involve colleagues, such as colorectal surgeons or urologists. For example, deep infiltrative endometriosis penetrating into the bowel mucosa will require a discoid or segmental bowel resection.
While many endometriosis experts rely on MRI, many MIG surgeons are dependent on ultrasound. I would not consider taking a patient with signs and symptoms suggestive of endometriosis to surgery without 2D/3D transvaginal ultrasound. If the patient possesses a uterus, a saline-infused sonogram is performed to potentially diagnose adenomyosis.
It is a pleasure and honor to welcome Professor Caterina Exacoustos MD, PhD, associate professor of ob.gyn. at the University of Rome “Tor Vergata,” to this edition of the Master Class in Gynecologic Surgery to discuss “Ultrasound and Its Role in the Diagnosis of and Management of Endometriosis, Including DIE.”
Prof. Exacoustos’ main areas of interest are endometriosis and benign diseases including uterine pathology and infertility. Her extensive body of work comprises over 120 scientific publications and numerous book chapters both in English and in Italian.
Prof. Exacoustos continues to be one of the most well respected lecturers speaking about ultrasound throughout the world.
Dr. Miller is professor of obstetrics and gynecology, department of clinical sciences, Rosalind Franklin University of Medicine and Science, North Chicago. Dr. Miller has no conflicts of interest to report.
Ultrasound and Its Role In Diagnosing and Managing Endometriosis
Endometriosis affects approximately 10%-20% of premenopausal women worldwide. It is the leading cause of chronic pelvic pain, is often associated with infertility, and has a significant impact on quality of life. Although the natural history of endometriosis remains unknown, emerging evidence suggests that the pathophysiological steps of initiation and development of endometriosis must occur earlier in the lifespan. Most notably, the onset of endometriosis-associated pain symptoms is often reported during adolescence and young adulthood.1
While many patients with endometriosis are referred with dysmenorrhea at a young age, at age ≤ 25 years,2 symptoms are often highly underestimated and considered to be normal and transient.3,4 Clinical and pelvic exams are often negative in young women, and delays in endometriosis diagnosis are well known.
The presentation of primary dysmenorrhea with no anatomical cause embodies the paradigm that dysmenorrhea in adolescents is most often an insignificant disorder. This perspective is probably a root cause of delayed endometriosis diagnosis in young patients. However, another issue behind delayed diagnosis is the reluctance of the physician to perform a diagnostic laparoscopy — historically the gold standard for diagnosing endometriosis — for seemingly common symptoms such as dysmenorrhea in young patients.
Today we know that there are typical aspects of ultrasound imaging that identify endometriosis in the pelvis, and notably, the 2022 European Society for Human Reproduction and Embryology (ESHRE) endometriosis guideline5 recognizes imaging (ultrasound or MRI) as the standard for endometriosis diagnosis without requiring laparoscopic or histological confirmation.
An early and noninvasive method of diagnosis aids in timely diagnosis and provides for the timely initiation of medical management to improve quality of life and prevent progression of disease (Figure 1).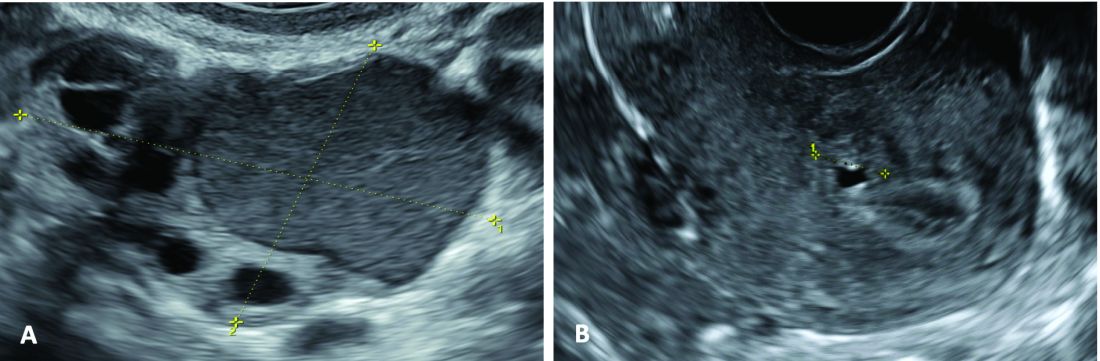
(A. Transvaginal ultrasound appearance of a small ovarian endometrioma in a 16-year-old girl. Note the unilocular cyst with ground glass echogenicity surrounded by multifollicular ovarian tissue. B. Ultrasound image of a retroverted uterus of an 18-year-old girl with focal adenomyosis of the posterior wall. Note the round cystic anechoic areas in the inner myometrium or junctional zone. The small intra-myometrial cyst is surrounded by a hyperechoic ring).
Indeed, the typical appearance of endometriotic pelvic lesions on transvaginal sonography, such as endometriomas and rectal deep infiltrating endometriosis (DIE) — as well as adenomyosis – can be medically treated without histologic confirmation .
When surgery is advisable, ultrasound findings also play a valuable role in presurgical staging, planning, and counseling for patients of all ages. Determining the extent and location of DIE preoperatively, for instance, facilitates the engagement of the appropriate surgical specialists so that multiple surgeries can be avoided. It also enables patients to be optimally informed before surgery of possible outcomes and complications.
Moreover, in the context of infertility, ultrasound can be a valuable tool for understanding uterine pathology and assessing for adenomyosis so that affected patients may be treated surgically or medically before turning to assisted reproductive technology.
Uniformity, Standardization in the Sonographic Assessment
In Europe, as in the United States, transvaginal sonography (TVS) is the first-line imaging tool for the diagnosis and management of endometriosis. In Europe, many ob.gyns. perform ultrasound themselves, as do treating surgeons. When diagnostic findings are negative but clinical suspicion is high, MRI is often utilized. Laparoscopy may then be considered in patients with negative imaging results.
Efforts to standardize terms, definitions, measurements, and sonographic features of different types of endometriosis have been made to make it easier for physicians to share data and communicate with each other. A lack of uniformity has contributed to variability in the reported diagnostic accuracy of TVS.
About 10 years ago, in one such effort, we assessed the accuracy of TVS for DIE by comparing TVS results with laparoscopic/histologic findings, and developed an ultrasound mapping system to accurately record the location, size and depth of lesions visualized by TVS. The accuracy of TVS ranged from 76% for the diagnosis of vaginal endometriosis to 97% for the diagnosis of bladder lesions and posterior cul-de-sac obliteration. Accuracy was 93% and 91% for detecting ureteral involvement (right and left); 87% for uterosacral ligament endometriotic lesions; and 87% for parametrial involvement.6
Shortly after, with a focus on DIE, expert sonographers and physician-sonographers from across Europe — as well as some experts from Australia, Japan, Brazil, Chile, and the United States (Y. Osuga from Brigham and Women’s Hospital and Harvard Medical School) — came together to agree on a uniform approach to the sonographic evaluation for suspected endometriosis and a standardization of terminology.
The consensus opinion from the International Deep Endometriosis Analysis (IDEA) group details four steps for examining women with suspected DIE: 1) Evaluation of the uterus and adnexa, 2) evaluation of transvaginal sonographic “soft markers” (ie. site-specific tenderness and ovarian mobility), 3) assessment of the status of the posterior cul-de-sac using real-time ultrasound-based “sliding sign,” and 4) assessment for DIE nodules in the anterior and posterior compartments.7
Our paper describing a mapping system and the IDEA paper describe how to detect deep endometriosis in the pelvis by utilizing an ultrasound view of normal anatomy and pelvic organ structure to provide landmarks for accurately defining the site of DIE lesions (Figure 2).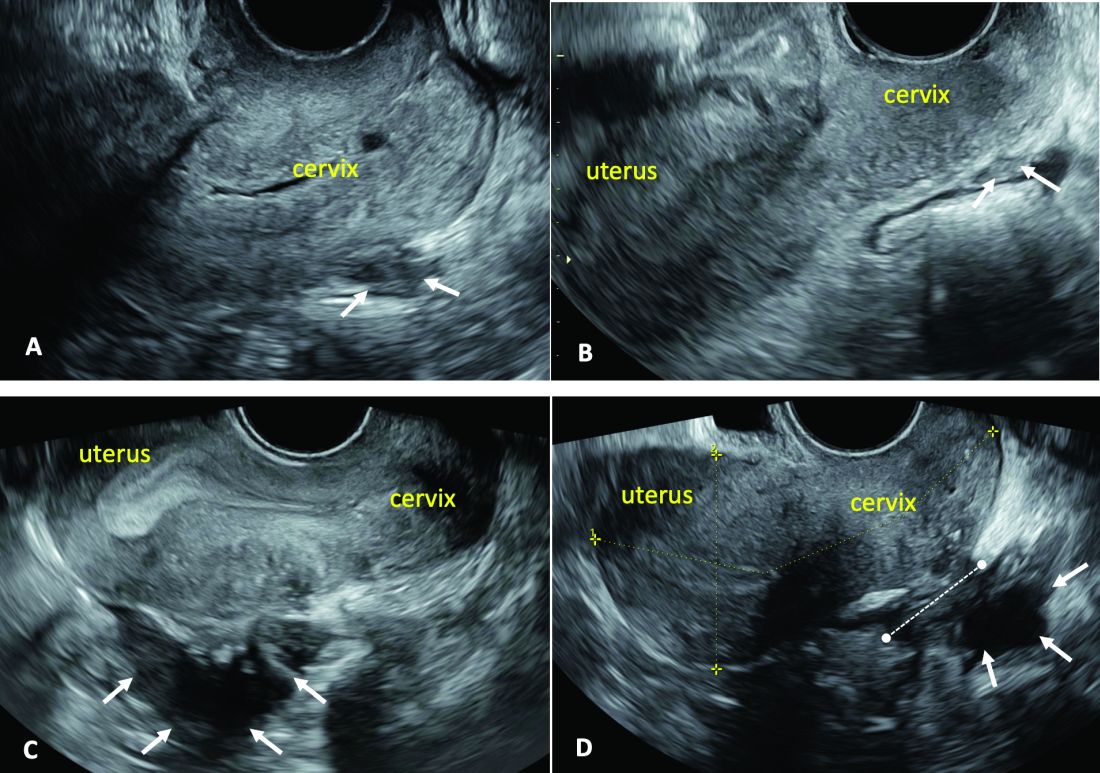
(A. Ultrasound appearance of a small DIE lesion of the retrocervical area [white arrows], which involved the torus uterinum and the right uterosacral ligament [USL]. The lesion appears as hypoechoic tissue with irregular margins caused by the fibrosis induced by the DIE. B. TVS appearance of small nodules of DIE of the left USL. Note the small retrocervical DIE lesion [white arrows], which appears hypoechoic due to the infiltration of the hyperechoic USL. C) Ultrasound appearance of a DIE nodule of the recto-sigmoid wall. Note the hypoechoic thickening of the muscular layers of the bowel wall attached to the corpus of the uterus and the adenomyosis of the posterior wall. The retrocervical area is free. D. TVS appearance of nodules of DIE of the lower rectal wall. Note the hypoechoic lesion [white arrows] of the rectum is attached to a retrocervical DIE fibrosis of the torus and USL [white dotted line]).
So-called rectovaginal endometriosis can be well assessed, for instance, since the involvement of the rectum, sigmoid colon, vaginal wall, rectovaginal septum, and posterior cul-de-sac uterosacral ligament can be seen by ultrasound as a single structure, making the location, size, and depth of any lesions discernible.
Again, this evaluation of the extent of disease is important for presurgical assessment so the surgeon can organize the right team and time of surgery and so the patient can be counseled on the advantages and possible complications of the treatment.
Notably, an accurate ultrasound description of pelvic endometriosis is helpful for accurate classification of disease. Endometriosis classification systems such as that of the American Association of Gynecologic Laparoscopists (AAGL)8 and the American Society of Reproductive Medicine (ASRM),9 as well as the #Enzian surgical description system,10 have been adapted to cover findings from ultrasound as well as MRI imaging.
A Systematic Evaluation
In keeping with the IDEA consensus opinion and based on our years of experience at the University of Rome, I advise that patients with typical pain symptoms of endometriosis or infertility undergo an accurate sonographic assessment of the pelvis with particular evaluation not only of the uterus and ovaries but of all pelvic retroperitoneal spaces.
The TVS examination should start with a slightly filled bladder, which permits a better evaluation of the bladder walls and the presence of endometriotic nodules. These nodules appear as hyperechoic linear or spherical lesions bulging toward the lumen and involving the serosa, muscularis, or (sub)mucosa of the bladder.
Then, an accurate evaluation of the uterus in 2D and 3D permits the diagnosis of adenomyosis. 3D sonographic evaluation of the myometrium and of the junctional zone are important; alteration and infiltration of the junctional zone and the presence of small adenomyotic cysts in the inner or outer myometrium are direct, specific signs of adenomyosis and should be ruled out in patients with dysmenorrhea, heavy menstrual bleeding, infertility, and pregnancy complications.
Endometriomas of the ovaries can be easily detected as having the typical appearance of a cyst with ground glass content. Adhesions of the ovaries and the uterus also should be evaluated with a dynamic ultrasound approach that utilizes the sliding sign and mobilization by palpation of the organs during the TVS scan.
Finally, the posterior and lateral retroperitoneal compartments should be carefully evaluated, with symptoms guiding the TVS examination whenever possible. Deep endometriotic nodules of the rectum appear as hypoechoic lesions or linear or nodular retroperitoneal thickening with irregular borders, penetrating into the intestinal wall and distorting its normal structure. In young patients, it seems very important to assess for small lesions below the peritoneum between the vagina and rectum, and in the parametria and around the ureter and nerves — lesions that, notably, would not be seen by diagnostic laparoscopy.
The Evaluation of Young Patients
In adolescent and young patients, endometriosis and adenomyosis are often present with small lesions and shallow tissue invasion, making a very careful and experienced approach to ultrasound essential for detection. Endometriomas are often of small diameter, and DIE is not always easily diagnosed because retroperitoneal lesions are similarly very small.
In a series of 270 adolescents (ages 12-20) who were referred to our outpatient gynecologic ultrasound unit over a 5-year period for various indications, at least one ultrasound feature of endometriosis was observed in 13.3%. In those with dysmenorrhea, the detection of endometriosis increased to 21%. Endometrioma was the most common type of endometriosis we found in the study, but DIE and adenomyosis were found in 4%-11%.
Although endometriotic lesions typically are small in young patients, they are often associated with severe pain symptoms, including chronic pelvic pain, dysmenorrhea, dyspareunia, dysuria, and dyschezia, all of which can have a serious effect on the quality of life of these young women. These symptoms keep them away from school during menstruation, away from sports, and cause painful intercourse and infertility. In young patients, an accurate TVS can provide a lot of information, and the ability to detect retroperitoneal endometriotic lesions and adenomyosis is probably better than with purely diagnostic laparoscopy, which would evaluate only superficial lesions.
TVS or, when needed, transrectal ultrasound, can enable adequate treatment and follow-up of the disease and its symptoms. There are no guidelines recommending adequate follow-up times to evaluate the effectiveness of medical therapy in patients with ultrasound signs of endometriosis. (Likewise, there are no indications for follow-up in patients with severe dysmenorrhea without ultrasound signs of endometriosis.) Certainly, our studies suggest careful evaluation over time of young patients with severe dysmenorrhea by serial ultrasound scans. With such follow-up, disease progress can be monitored and the medical or surgical treatment approach modified if needed.
The diagnosis of endometriosis at a young age has significant benefits not only in avoiding or reducing progression of the disease, but also in improving quality of life and aiding women in their desire for pregnancy.
Dr. Exacoustos is associate professor of ob.gyn. at the University of Rome “Tor Vergata.” She has no conflicts of interest to report.
References
1. Zondervan KT et al. N Engl J Med. 2020;382:1244-56.
2. Greene R et al. Fertil Steril. 2009;91:32-9.
3. Chapron C et al. J Pediatr Adolesc Gynecol. 2011;24:S7-12.
4. Randhawa AE et al. J Pediatr Adolesc Gynecol. 2021;34:643-8.
5. Becker CM et al. Hum Reprod Open. 2022(2):hoac009.
6. Exacoustos C et al. Fertil Steril. 2014;102:143-9. 7. Guerriero S et al. Ultrasound Obstet Gynecol. 2016;48(3):318-32.
8. Abrao MS et al. J Minim Invasive Gynecol. 2021;28:1941-50.9. Revised American Society for Reproductive Medicine classification of endometriosis: 1996. Fertil Steril. 1997;67:817-21. 10. Keckstein J et al. Acta Obstet Gynecol Scand. 2021;100:1165-75.
11. Martire FG et al. Fertil Steril. 2020;114(5):1049-57.
Introduction: Imaging for Endometriosis — A Necessary Prerequisite
While the gold standard in the diagnosis of endometriosis remains laparoscopy, it is now recognized that thorough evaluation via ultrasound offers an acceptable, less expensive, and less invasive alternative. It is especially useful for the diagnosis of deep infiltrative disease, which penetrates more than 5 mm into the peritoneum, ovarian endometrioma, and when anatomic distortion occurs, such as to the path of the ureter.
Besides establishing the diagnosis, ultrasound imaging has become, along with MRI, the most important aid for proper preoperative planning. Not only does imaging provide the surgeon and patient with knowledge regarding the extent of the upcoming procedure, but it also allows the minimally invasive gynecologic (MIG) surgeon to involve colleagues, such as colorectal surgeons or urologists. For example, deep infiltrative endometriosis penetrating into the bowel mucosa will require a discoid or segmental bowel resection.
While many endometriosis experts rely on MRI, many MIG surgeons are dependent on ultrasound. I would not consider taking a patient with signs and symptoms suggestive of endometriosis to surgery without 2D/3D transvaginal ultrasound. If the patient possesses a uterus, a saline-infused sonogram is performed to potentially diagnose adenomyosis.
It is a pleasure and honor to welcome Professor Caterina Exacoustos MD, PhD, associate professor of ob.gyn. at the University of Rome “Tor Vergata,” to this edition of the Master Class in Gynecologic Surgery to discuss “Ultrasound and Its Role in the Diagnosis of and Management of Endometriosis, Including DIE.”
Prof. Exacoustos’ main areas of interest are endometriosis and benign diseases including uterine pathology and infertility. Her extensive body of work comprises over 120 scientific publications and numerous book chapters both in English and in Italian.
Prof. Exacoustos continues to be one of the most well respected lecturers speaking about ultrasound throughout the world.
Dr. Miller is professor of obstetrics and gynecology, department of clinical sciences, Rosalind Franklin University of Medicine and Science, North Chicago. Dr. Miller has no conflicts of interest to report.
Ultrasound and Its Role In Diagnosing and Managing Endometriosis
Endometriosis affects approximately 10%-20% of premenopausal women worldwide. It is the leading cause of chronic pelvic pain, is often associated with infertility, and has a significant impact on quality of life. Although the natural history of endometriosis remains unknown, emerging evidence suggests that the pathophysiological steps of initiation and development of endometriosis must occur earlier in the lifespan. Most notably, the onset of endometriosis-associated pain symptoms is often reported during adolescence and young adulthood.1
While many patients with endometriosis are referred with dysmenorrhea at a young age, at age ≤ 25 years,2 symptoms are often highly underestimated and considered to be normal and transient.3,4 Clinical and pelvic exams are often negative in young women, and delays in endometriosis diagnosis are well known.
The presentation of primary dysmenorrhea with no anatomical cause embodies the paradigm that dysmenorrhea in adolescents is most often an insignificant disorder. This perspective is probably a root cause of delayed endometriosis diagnosis in young patients. However, another issue behind delayed diagnosis is the reluctance of the physician to perform a diagnostic laparoscopy — historically the gold standard for diagnosing endometriosis — for seemingly common symptoms such as dysmenorrhea in young patients.
Today we know that there are typical aspects of ultrasound imaging that identify endometriosis in the pelvis, and notably, the 2022 European Society for Human Reproduction and Embryology (ESHRE) endometriosis guideline5 recognizes imaging (ultrasound or MRI) as the standard for endometriosis diagnosis without requiring laparoscopic or histological confirmation.
An early and noninvasive method of diagnosis aids in timely diagnosis and provides for the timely initiation of medical management to improve quality of life and prevent progression of disease (Figure 1).
(A. Transvaginal ultrasound appearance of a small ovarian endometrioma in a 16-year-old girl. Note the unilocular cyst with ground glass echogenicity surrounded by multifollicular ovarian tissue. B. Ultrasound image of a retroverted uterus of an 18-year-old girl with focal adenomyosis of the posterior wall. Note the round cystic anechoic areas in the inner myometrium or junctional zone. The small intra-myometrial cyst is surrounded by a hyperechoic ring).
Indeed, the typical appearance of endometriotic pelvic lesions on transvaginal sonography, such as endometriomas and rectal deep infiltrating endometriosis (DIE) — as well as adenomyosis – can be medically treated without histologic confirmation .
When surgery is advisable, ultrasound findings also play a valuable role in presurgical staging, planning, and counseling for patients of all ages. Determining the extent and location of DIE preoperatively, for instance, facilitates the engagement of the appropriate surgical specialists so that multiple surgeries can be avoided. It also enables patients to be optimally informed before surgery of possible outcomes and complications.
Moreover, in the context of infertility, ultrasound can be a valuable tool for understanding uterine pathology and assessing for adenomyosis so that affected patients may be treated surgically or medically before turning to assisted reproductive technology.
Uniformity, Standardization in the Sonographic Assessment
In Europe, as in the United States, transvaginal sonography (TVS) is the first-line imaging tool for the diagnosis and management of endometriosis. In Europe, many ob.gyns. perform ultrasound themselves, as do treating surgeons. When diagnostic findings are negative but clinical suspicion is high, MRI is often utilized. Laparoscopy may then be considered in patients with negative imaging results.
Efforts to standardize terms, definitions, measurements, and sonographic features of different types of endometriosis have been made to make it easier for physicians to share data and communicate with each other. A lack of uniformity has contributed to variability in the reported diagnostic accuracy of TVS.
About 10 years ago, in one such effort, we assessed the accuracy of TVS for DIE by comparing TVS results with laparoscopic/histologic findings, and developed an ultrasound mapping system to accurately record the location, size and depth of lesions visualized by TVS. The accuracy of TVS ranged from 76% for the diagnosis of vaginal endometriosis to 97% for the diagnosis of bladder lesions and posterior cul-de-sac obliteration. Accuracy was 93% and 91% for detecting ureteral involvement (right and left); 87% for uterosacral ligament endometriotic lesions; and 87% for parametrial involvement.6
Shortly after, with a focus on DIE, expert sonographers and physician-sonographers from across Europe — as well as some experts from Australia, Japan, Brazil, Chile, and the United States (Y. Osuga from Brigham and Women’s Hospital and Harvard Medical School) — came together to agree on a uniform approach to the sonographic evaluation for suspected endometriosis and a standardization of terminology.
The consensus opinion from the International Deep Endometriosis Analysis (IDEA) group details four steps for examining women with suspected DIE: 1) Evaluation of the uterus and adnexa, 2) evaluation of transvaginal sonographic “soft markers” (ie. site-specific tenderness and ovarian mobility), 3) assessment of the status of the posterior cul-de-sac using real-time ultrasound-based “sliding sign,” and 4) assessment for DIE nodules in the anterior and posterior compartments.7
Our paper describing a mapping system and the IDEA paper describe how to detect deep endometriosis in the pelvis by utilizing an ultrasound view of normal anatomy and pelvic organ structure to provide landmarks for accurately defining the site of DIE lesions (Figure 2).
(A. Ultrasound appearance of a small DIE lesion of the retrocervical area [white arrows], which involved the torus uterinum and the right uterosacral ligament [USL]. The lesion appears as hypoechoic tissue with irregular margins caused by the fibrosis induced by the DIE. B. TVS appearance of small nodules of DIE of the left USL. Note the small retrocervical DIE lesion [white arrows], which appears hypoechoic due to the infiltration of the hyperechoic USL. C) Ultrasound appearance of a DIE nodule of the recto-sigmoid wall. Note the hypoechoic thickening of the muscular layers of the bowel wall attached to the corpus of the uterus and the adenomyosis of the posterior wall. The retrocervical area is free. D. TVS appearance of nodules of DIE of the lower rectal wall. Note the hypoechoic lesion [white arrows] of the rectum is attached to a retrocervical DIE fibrosis of the torus and USL [white dotted line]).
So-called rectovaginal endometriosis can be well assessed, for instance, since the involvement of the rectum, sigmoid colon, vaginal wall, rectovaginal septum, and posterior cul-de-sac uterosacral ligament can be seen by ultrasound as a single structure, making the location, size, and depth of any lesions discernible.
Again, this evaluation of the extent of disease is important for presurgical assessment so the surgeon can organize the right team and time of surgery and so the patient can be counseled on the advantages and possible complications of the treatment.
Notably, an accurate ultrasound description of pelvic endometriosis is helpful for accurate classification of disease. Endometriosis classification systems such as that of the American Association of Gynecologic Laparoscopists (AAGL)8 and the American Society of Reproductive Medicine (ASRM),9 as well as the #Enzian surgical description system,10 have been adapted to cover findings from ultrasound as well as MRI imaging.
A Systematic Evaluation
In keeping with the IDEA consensus opinion and based on our years of experience at the University of Rome, I advise that patients with typical pain symptoms of endometriosis or infertility undergo an accurate sonographic assessment of the pelvis with particular evaluation not only of the uterus and ovaries but of all pelvic retroperitoneal spaces.
The TVS examination should start with a slightly filled bladder, which permits a better evaluation of the bladder walls and the presence of endometriotic nodules. These nodules appear as hyperechoic linear or spherical lesions bulging toward the lumen and involving the serosa, muscularis, or (sub)mucosa of the bladder.
Then, an accurate evaluation of the uterus in 2D and 3D permits the diagnosis of adenomyosis. 3D sonographic evaluation of the myometrium and of the junctional zone are important; alteration and infiltration of the junctional zone and the presence of small adenomyotic cysts in the inner or outer myometrium are direct, specific signs of adenomyosis and should be ruled out in patients with dysmenorrhea, heavy menstrual bleeding, infertility, and pregnancy complications.
Endometriomas of the ovaries can be easily detected as having the typical appearance of a cyst with ground glass content. Adhesions of the ovaries and the uterus also should be evaluated with a dynamic ultrasound approach that utilizes the sliding sign and mobilization by palpation of the organs during the TVS scan.
Finally, the posterior and lateral retroperitoneal compartments should be carefully evaluated, with symptoms guiding the TVS examination whenever possible. Deep endometriotic nodules of the rectum appear as hypoechoic lesions or linear or nodular retroperitoneal thickening with irregular borders, penetrating into the intestinal wall and distorting its normal structure. In young patients, it seems very important to assess for small lesions below the peritoneum between the vagina and rectum, and in the parametria and around the ureter and nerves — lesions that, notably, would not be seen by diagnostic laparoscopy.
The Evaluation of Young Patients
In adolescent and young patients, endometriosis and adenomyosis are often present with small lesions and shallow tissue invasion, making a very careful and experienced approach to ultrasound essential for detection. Endometriomas are often of small diameter, and DIE is not always easily diagnosed because retroperitoneal lesions are similarly very small.
In a series of 270 adolescents (ages 12-20) who were referred to our outpatient gynecologic ultrasound unit over a 5-year period for various indications, at least one ultrasound feature of endometriosis was observed in 13.3%. In those with dysmenorrhea, the detection of endometriosis increased to 21%. Endometrioma was the most common type of endometriosis we found in the study, but DIE and adenomyosis were found in 4%-11%.
Although endometriotic lesions typically are small in young patients, they are often associated with severe pain symptoms, including chronic pelvic pain, dysmenorrhea, dyspareunia, dysuria, and dyschezia, all of which can have a serious effect on the quality of life of these young women. These symptoms keep them away from school during menstruation, away from sports, and cause painful intercourse and infertility. In young patients, an accurate TVS can provide a lot of information, and the ability to detect retroperitoneal endometriotic lesions and adenomyosis is probably better than with purely diagnostic laparoscopy, which would evaluate only superficial lesions.
TVS or, when needed, transrectal ultrasound, can enable adequate treatment and follow-up of the disease and its symptoms. There are no guidelines recommending adequate follow-up times to evaluate the effectiveness of medical therapy in patients with ultrasound signs of endometriosis. (Likewise, there are no indications for follow-up in patients with severe dysmenorrhea without ultrasound signs of endometriosis.) Certainly, our studies suggest careful evaluation over time of young patients with severe dysmenorrhea by serial ultrasound scans. With such follow-up, disease progress can be monitored and the medical or surgical treatment approach modified if needed.
The diagnosis of endometriosis at a young age has significant benefits not only in avoiding or reducing progression of the disease, but also in improving quality of life and aiding women in their desire for pregnancy.
Dr. Exacoustos is associate professor of ob.gyn. at the University of Rome “Tor Vergata.” She has no conflicts of interest to report.
References
1. Zondervan KT et al. N Engl J Med. 2020;382:1244-56.
2. Greene R et al. Fertil Steril. 2009;91:32-9.
3. Chapron C et al. J Pediatr Adolesc Gynecol. 2011;24:S7-12.
4. Randhawa AE et al. J Pediatr Adolesc Gynecol. 2021;34:643-8.
5. Becker CM et al. Hum Reprod Open. 2022(2):hoac009.
6. Exacoustos C et al. Fertil Steril. 2014;102:143-9. 7. Guerriero S et al. Ultrasound Obstet Gynecol. 2016;48(3):318-32.
8. Abrao MS et al. J Minim Invasive Gynecol. 2021;28:1941-50.9. Revised American Society for Reproductive Medicine classification of endometriosis: 1996. Fertil Steril. 1997;67:817-21. 10. Keckstein J et al. Acta Obstet Gynecol Scand. 2021;100:1165-75.
11. Martire FG et al. Fertil Steril. 2020;114(5):1049-57.
Introduction: Imaging for Endometriosis — A Necessary Prerequisite
While the gold standard in the diagnosis of endometriosis remains laparoscopy, it is now recognized that thorough evaluation via ultrasound offers an acceptable, less expensive, and less invasive alternative. It is especially useful for the diagnosis of deep infiltrative disease, which penetrates more than 5 mm into the peritoneum, ovarian endometrioma, and when anatomic distortion occurs, such as to the path of the ureter.
Besides establishing the diagnosis, ultrasound imaging has become, along with MRI, the most important aid for proper preoperative planning. Not only does imaging provide the surgeon and patient with knowledge regarding the extent of the upcoming procedure, but it also allows the minimally invasive gynecologic (MIG) surgeon to involve colleagues, such as colorectal surgeons or urologists. For example, deep infiltrative endometriosis penetrating into the bowel mucosa will require a discoid or segmental bowel resection.
While many endometriosis experts rely on MRI, many MIG surgeons are dependent on ultrasound. I would not consider taking a patient with signs and symptoms suggestive of endometriosis to surgery without 2D/3D transvaginal ultrasound. If the patient possesses a uterus, a saline-infused sonogram is performed to potentially diagnose adenomyosis.
It is a pleasure and honor to welcome Professor Caterina Exacoustos MD, PhD, associate professor of ob.gyn. at the University of Rome “Tor Vergata,” to this edition of the Master Class in Gynecologic Surgery to discuss “Ultrasound and Its Role in the Diagnosis of and Management of Endometriosis, Including DIE.”
Prof. Exacoustos’ main areas of interest are endometriosis and benign diseases including uterine pathology and infertility. Her extensive body of work comprises over 120 scientific publications and numerous book chapters both in English and in Italian.
Prof. Exacoustos continues to be one of the most well respected lecturers speaking about ultrasound throughout the world.
Dr. Miller is professor of obstetrics and gynecology, department of clinical sciences, Rosalind Franklin University of Medicine and Science, North Chicago. Dr. Miller has no conflicts of interest to report.
Ultrasound and Its Role In Diagnosing and Managing Endometriosis
Endometriosis affects approximately 10%-20% of premenopausal women worldwide. It is the leading cause of chronic pelvic pain, is often associated with infertility, and has a significant impact on quality of life. Although the natural history of endometriosis remains unknown, emerging evidence suggests that the pathophysiological steps of initiation and development of endometriosis must occur earlier in the lifespan. Most notably, the onset of endometriosis-associated pain symptoms is often reported during adolescence and young adulthood.1
While many patients with endometriosis are referred with dysmenorrhea at a young age, at age ≤ 25 years,2 symptoms are often highly underestimated and considered to be normal and transient.3,4 Clinical and pelvic exams are often negative in young women, and delays in endometriosis diagnosis are well known.
The presentation of primary dysmenorrhea with no anatomical cause embodies the paradigm that dysmenorrhea in adolescents is most often an insignificant disorder. This perspective is probably a root cause of delayed endometriosis diagnosis in young patients. However, another issue behind delayed diagnosis is the reluctance of the physician to perform a diagnostic laparoscopy — historically the gold standard for diagnosing endometriosis — for seemingly common symptoms such as dysmenorrhea in young patients.
Today we know that there are typical aspects of ultrasound imaging that identify endometriosis in the pelvis, and notably, the 2022 European Society for Human Reproduction and Embryology (ESHRE) endometriosis guideline5 recognizes imaging (ultrasound or MRI) as the standard for endometriosis diagnosis without requiring laparoscopic or histological confirmation.
An early and noninvasive method of diagnosis aids in timely diagnosis and provides for the timely initiation of medical management to improve quality of life and prevent progression of disease (Figure 1).
(A. Transvaginal ultrasound appearance of a small ovarian endometrioma in a 16-year-old girl. Note the unilocular cyst with ground glass echogenicity surrounded by multifollicular ovarian tissue. B. Ultrasound image of a retroverted uterus of an 18-year-old girl with focal adenomyosis of the posterior wall. Note the round cystic anechoic areas in the inner myometrium or junctional zone. The small intra-myometrial cyst is surrounded by a hyperechoic ring).
Indeed, the typical appearance of endometriotic pelvic lesions on transvaginal sonography, such as endometriomas and rectal deep infiltrating endometriosis (DIE) — as well as adenomyosis – can be medically treated without histologic confirmation .
When surgery is advisable, ultrasound findings also play a valuable role in presurgical staging, planning, and counseling for patients of all ages. Determining the extent and location of DIE preoperatively, for instance, facilitates the engagement of the appropriate surgical specialists so that multiple surgeries can be avoided. It also enables patients to be optimally informed before surgery of possible outcomes and complications.
Moreover, in the context of infertility, ultrasound can be a valuable tool for understanding uterine pathology and assessing for adenomyosis so that affected patients may be treated surgically or medically before turning to assisted reproductive technology.
Uniformity, Standardization in the Sonographic Assessment
In Europe, as in the United States, transvaginal sonography (TVS) is the first-line imaging tool for the diagnosis and management of endometriosis. In Europe, many ob.gyns. perform ultrasound themselves, as do treating surgeons. When diagnostic findings are negative but clinical suspicion is high, MRI is often utilized. Laparoscopy may then be considered in patients with negative imaging results.
Efforts to standardize terms, definitions, measurements, and sonographic features of different types of endometriosis have been made to make it easier for physicians to share data and communicate with each other. A lack of uniformity has contributed to variability in the reported diagnostic accuracy of TVS.
About 10 years ago, in one such effort, we assessed the accuracy of TVS for DIE by comparing TVS results with laparoscopic/histologic findings, and developed an ultrasound mapping system to accurately record the location, size and depth of lesions visualized by TVS. The accuracy of TVS ranged from 76% for the diagnosis of vaginal endometriosis to 97% for the diagnosis of bladder lesions and posterior cul-de-sac obliteration. Accuracy was 93% and 91% for detecting ureteral involvement (right and left); 87% for uterosacral ligament endometriotic lesions; and 87% for parametrial involvement.6
Shortly after, with a focus on DIE, expert sonographers and physician-sonographers from across Europe — as well as some experts from Australia, Japan, Brazil, Chile, and the United States (Y. Osuga from Brigham and Women’s Hospital and Harvard Medical School) — came together to agree on a uniform approach to the sonographic evaluation for suspected endometriosis and a standardization of terminology.
The consensus opinion from the International Deep Endometriosis Analysis (IDEA) group details four steps for examining women with suspected DIE: 1) Evaluation of the uterus and adnexa, 2) evaluation of transvaginal sonographic “soft markers” (ie. site-specific tenderness and ovarian mobility), 3) assessment of the status of the posterior cul-de-sac using real-time ultrasound-based “sliding sign,” and 4) assessment for DIE nodules in the anterior and posterior compartments.7
Our paper describing a mapping system and the IDEA paper describe how to detect deep endometriosis in the pelvis by utilizing an ultrasound view of normal anatomy and pelvic organ structure to provide landmarks for accurately defining the site of DIE lesions (Figure 2).
(A. Ultrasound appearance of a small DIE lesion of the retrocervical area [white arrows], which involved the torus uterinum and the right uterosacral ligament [USL]. The lesion appears as hypoechoic tissue with irregular margins caused by the fibrosis induced by the DIE. B. TVS appearance of small nodules of DIE of the left USL. Note the small retrocervical DIE lesion [white arrows], which appears hypoechoic due to the infiltration of the hyperechoic USL. C) Ultrasound appearance of a DIE nodule of the recto-sigmoid wall. Note the hypoechoic thickening of the muscular layers of the bowel wall attached to the corpus of the uterus and the adenomyosis of the posterior wall. The retrocervical area is free. D. TVS appearance of nodules of DIE of the lower rectal wall. Note the hypoechoic lesion [white arrows] of the rectum is attached to a retrocervical DIE fibrosis of the torus and USL [white dotted line]).
So-called rectovaginal endometriosis can be well assessed, for instance, since the involvement of the rectum, sigmoid colon, vaginal wall, rectovaginal septum, and posterior cul-de-sac uterosacral ligament can be seen by ultrasound as a single structure, making the location, size, and depth of any lesions discernible.
Again, this evaluation of the extent of disease is important for presurgical assessment so the surgeon can organize the right team and time of surgery and so the patient can be counseled on the advantages and possible complications of the treatment.
Notably, an accurate ultrasound description of pelvic endometriosis is helpful for accurate classification of disease. Endometriosis classification systems such as that of the American Association of Gynecologic Laparoscopists (AAGL)8 and the American Society of Reproductive Medicine (ASRM),9 as well as the #Enzian surgical description system,10 have been adapted to cover findings from ultrasound as well as MRI imaging.
A Systematic Evaluation
In keeping with the IDEA consensus opinion and based on our years of experience at the University of Rome, I advise that patients with typical pain symptoms of endometriosis or infertility undergo an accurate sonographic assessment of the pelvis with particular evaluation not only of the uterus and ovaries but of all pelvic retroperitoneal spaces.
The TVS examination should start with a slightly filled bladder, which permits a better evaluation of the bladder walls and the presence of endometriotic nodules. These nodules appear as hyperechoic linear or spherical lesions bulging toward the lumen and involving the serosa, muscularis, or (sub)mucosa of the bladder.
Then, an accurate evaluation of the uterus in 2D and 3D permits the diagnosis of adenomyosis. 3D sonographic evaluation of the myometrium and of the junctional zone are important; alteration and infiltration of the junctional zone and the presence of small adenomyotic cysts in the inner or outer myometrium are direct, specific signs of adenomyosis and should be ruled out in patients with dysmenorrhea, heavy menstrual bleeding, infertility, and pregnancy complications.
Endometriomas of the ovaries can be easily detected as having the typical appearance of a cyst with ground glass content. Adhesions of the ovaries and the uterus also should be evaluated with a dynamic ultrasound approach that utilizes the sliding sign and mobilization by palpation of the organs during the TVS scan.
Finally, the posterior and lateral retroperitoneal compartments should be carefully evaluated, with symptoms guiding the TVS examination whenever possible. Deep endometriotic nodules of the rectum appear as hypoechoic lesions or linear or nodular retroperitoneal thickening with irregular borders, penetrating into the intestinal wall and distorting its normal structure. In young patients, it seems very important to assess for small lesions below the peritoneum between the vagina and rectum, and in the parametria and around the ureter and nerves — lesions that, notably, would not be seen by diagnostic laparoscopy.
The Evaluation of Young Patients
In adolescent and young patients, endometriosis and adenomyosis are often present with small lesions and shallow tissue invasion, making a very careful and experienced approach to ultrasound essential for detection. Endometriomas are often of small diameter, and DIE is not always easily diagnosed because retroperitoneal lesions are similarly very small.
In a series of 270 adolescents (ages 12-20) who were referred to our outpatient gynecologic ultrasound unit over a 5-year period for various indications, at least one ultrasound feature of endometriosis was observed in 13.3%. In those with dysmenorrhea, the detection of endometriosis increased to 21%. Endometrioma was the most common type of endometriosis we found in the study, but DIE and adenomyosis were found in 4%-11%.
Although endometriotic lesions typically are small in young patients, they are often associated with severe pain symptoms, including chronic pelvic pain, dysmenorrhea, dyspareunia, dysuria, and dyschezia, all of which can have a serious effect on the quality of life of these young women. These symptoms keep them away from school during menstruation, away from sports, and cause painful intercourse and infertility. In young patients, an accurate TVS can provide a lot of information, and the ability to detect retroperitoneal endometriotic lesions and adenomyosis is probably better than with purely diagnostic laparoscopy, which would evaluate only superficial lesions.
TVS or, when needed, transrectal ultrasound, can enable adequate treatment and follow-up of the disease and its symptoms. There are no guidelines recommending adequate follow-up times to evaluate the effectiveness of medical therapy in patients with ultrasound signs of endometriosis. (Likewise, there are no indications for follow-up in patients with severe dysmenorrhea without ultrasound signs of endometriosis.) Certainly, our studies suggest careful evaluation over time of young patients with severe dysmenorrhea by serial ultrasound scans. With such follow-up, disease progress can be monitored and the medical or surgical treatment approach modified if needed.
The diagnosis of endometriosis at a young age has significant benefits not only in avoiding or reducing progression of the disease, but also in improving quality of life and aiding women in their desire for pregnancy.
Dr. Exacoustos is associate professor of ob.gyn. at the University of Rome “Tor Vergata.” She has no conflicts of interest to report.
References
1. Zondervan KT et al. N Engl J Med. 2020;382:1244-56.
2. Greene R et al. Fertil Steril. 2009;91:32-9.
3. Chapron C et al. J Pediatr Adolesc Gynecol. 2011;24:S7-12.
4. Randhawa AE et al. J Pediatr Adolesc Gynecol. 2021;34:643-8.
5. Becker CM et al. Hum Reprod Open. 2022(2):hoac009.
6. Exacoustos C et al. Fertil Steril. 2014;102:143-9. 7. Guerriero S et al. Ultrasound Obstet Gynecol. 2016;48(3):318-32.
8. Abrao MS et al. J Minim Invasive Gynecol. 2021;28:1941-50.9. Revised American Society for Reproductive Medicine classification of endometriosis: 1996. Fertil Steril. 1997;67:817-21. 10. Keckstein J et al. Acta Obstet Gynecol Scand. 2021;100:1165-75.
11. Martire FG et al. Fertil Steril. 2020;114(5):1049-57.
AI and Suicide Prevention in Primary Care: A Q&A
Primary care physicians play a critical role in identifying patients at risk for serious mental health issues, including suicidality. But the ever-increasing demands on their clinical time can hinder the ability to identify emotional distress in time to intervene. Can artificial intelligence (AI) help?
This news organization spoke with Tom Zaubler, MD, a psychiatrist and chief medical officer of NeuroFlow, about how AI can improve the ability of primary care physicians and other clinicians to screen their patients for suicidal ideation and boost rates of treatment for mental health issues in their patients. This interview has been edited for clarity and length.
Question: How can AI help in suicide prevention and mental health screening in primary care?
Answer: Recent studies have demonstrated the potential of AI in mental health screening and suicide prevention. One method is natural language processing (NLP), which can analyze patients› journal entries for signs of suicidal thoughts or behaviors. This technology has shown promise in detecting suicidal ideation in patients who may not report such thoughts on traditional screening tools like the Patient Health Questionnaire-9 (PHQ-9). AI can be part of an integrated approach to identify and provide support to individuals at risk for suicide or those without a psychiatric history but who may still be at risk.
Q: A recent study by [Maria] Oquendo and colleagues found that one fifth of patients who attempt suicide do not meet the criteria for a mental health disorder.
Improved screening is obviously important, but in some ways it’s not the most important part of the problem. The lack of accessibility to specialized mental health care is a critical obstacle to treating patients with acute psychiatric needs.
How can primary care doctors effectively connect patients with mental health support, given the scarcity of mental health professionals?
A: Primary care doctors can leverage technology to extend mental health support. This includes using platforms for safety screening and providing patients with immediate access to local and national resources and digital interventions. Alerts can be sent to professionals within the practice or employed by technology companies to offer immediate support, including suicide safety planning and counseling. Users can hit a button to “Find a Therapist.” Also, if they acknowledge feelings of self-harm, these keywords are detected within the app by NLP. “Urgent alerts” are then sent to clinicians who are overseeing patient care. If someone is flagged, a social worker or member of a response services team intervenes and calls the person at risk to tailor care. These interventions do not always require a psychiatrist or masters-prepared clinician but can be effectively managed by trained paraprofessionals. These staff members can provide suicide safety planning and lethal-means-restriction counseling, and can assess the need for escalation of care.
Q: How is technology likely to manifest in physician practices in the near future to support mental health care?
A: Automated screening platforms for depression and anxiety, alerts for physicians when patients screen positively, and integration with collaborative care models are a few of the ways technology will become part of clinical practice. Additionally, advanced data analytics and predictive modeling using electronic health records and claims data will help identify high-risk patients. Technologies like voice recognition and machine learning can analyze patient journals and possibly, in the future, social media feeds to detect mental health issues. These technologies aim to extend and augment the capabilities of healthcare practices, improving the identification and management of patients at risk for mental health issues.
Q: Are these technologies as effective in pediatric populations, and are there any specific challenges?
A: Technologies for mental health screening and support are effective in pediatric populations, with certain age-specific considerations and legal restrictions on technology use. For adolescents and older children comfortable with technology, digital tools can significantly impact mental health care. For younger children, technology must facilitate information-gathering from various sources, including parents and teachers. Despite challenges, technology is crucial for early identification and intervention in pediatric mental health, potentially shortening the time to diagnosis and improving outcomes.
The statistics are horrifying. One third of adolescent girls have seriously thought about suicide over the past year; 13% attempt suicide. So there’s a need in the adolescent population and in the preadolescent population, too, because there’s an 8- to 10-year lag between onset of symptoms and diagnosis of mental illness. If we can shorten that lag, you see improved performance in schools; you see decreased truancy; you see greater economic achievement and so on. It makes such a profound difference. Not to mention it saves lives. So, yes, technology is critical in a pediatric population. It exists and it’s happening right now. There are challenges, but the goal can be met.
Q: A 2014 study found that 45% of people who completed suicide visited a primary care physician in the preceding month. And only 23% of people who attempt suicide have not seen a primary care physician within the past year. What does that say about the importance of screening at the primary care level?
A: The fact that a significant percentage of individuals who die by suicide have visited a primary care physician within a month or year prior to their death underscores the critical role of primary care in suicide prevention. This highlights the potential for primary care settings to identify and intervene with individuals at risk for suicide, making the case for the importance of integrating effective mental health screenings and support technologies in primary care practices.
Q: In other words, we’re not talking about a marginal benefit.
A: No, the potential benefit is huge. The United States Preventive Services Task Force did not endorse universal screening for suicide in its 2023 recommendations; they felt — and I accept that conclusion — there wasn›t enough evidence [at the time] to really support that recommendation. I think when you talk to a lot of suicide researchers, what you will hear is that providing suicide assessments as far upstream as possible is critical, especially when you start seeing more and more research showing that 20% of the population who die by suicide are not likely to have any psychiatric pathology at all. I believe the evidence base will soon support a recommendation for universal screening for adults. I believe it is especially important to screen for suicidal ideation in kids, given the high rates of suicide in this population.
Dr. Zaubler has disclosed the following relevant financial relationships: chief medical officer, NeuroFlow.
A version of this article appeared on Medscape.com.
Primary care physicians play a critical role in identifying patients at risk for serious mental health issues, including suicidality. But the ever-increasing demands on their clinical time can hinder the ability to identify emotional distress in time to intervene. Can artificial intelligence (AI) help?
This news organization spoke with Tom Zaubler, MD, a psychiatrist and chief medical officer of NeuroFlow, about how AI can improve the ability of primary care physicians and other clinicians to screen their patients for suicidal ideation and boost rates of treatment for mental health issues in their patients. This interview has been edited for clarity and length.
Question: How can AI help in suicide prevention and mental health screening in primary care?
Answer: Recent studies have demonstrated the potential of AI in mental health screening and suicide prevention. One method is natural language processing (NLP), which can analyze patients› journal entries for signs of suicidal thoughts or behaviors. This technology has shown promise in detecting suicidal ideation in patients who may not report such thoughts on traditional screening tools like the Patient Health Questionnaire-9 (PHQ-9). AI can be part of an integrated approach to identify and provide support to individuals at risk for suicide or those without a psychiatric history but who may still be at risk.
Q: A recent study by [Maria] Oquendo and colleagues found that one fifth of patients who attempt suicide do not meet the criteria for a mental health disorder.
Improved screening is obviously important, but in some ways it’s not the most important part of the problem. The lack of accessibility to specialized mental health care is a critical obstacle to treating patients with acute psychiatric needs.
How can primary care doctors effectively connect patients with mental health support, given the scarcity of mental health professionals?
A: Primary care doctors can leverage technology to extend mental health support. This includes using platforms for safety screening and providing patients with immediate access to local and national resources and digital interventions. Alerts can be sent to professionals within the practice or employed by technology companies to offer immediate support, including suicide safety planning and counseling. Users can hit a button to “Find a Therapist.” Also, if they acknowledge feelings of self-harm, these keywords are detected within the app by NLP. “Urgent alerts” are then sent to clinicians who are overseeing patient care. If someone is flagged, a social worker or member of a response services team intervenes and calls the person at risk to tailor care. These interventions do not always require a psychiatrist or masters-prepared clinician but can be effectively managed by trained paraprofessionals. These staff members can provide suicide safety planning and lethal-means-restriction counseling, and can assess the need for escalation of care.
Q: How is technology likely to manifest in physician practices in the near future to support mental health care?
A: Automated screening platforms for depression and anxiety, alerts for physicians when patients screen positively, and integration with collaborative care models are a few of the ways technology will become part of clinical practice. Additionally, advanced data analytics and predictive modeling using electronic health records and claims data will help identify high-risk patients. Technologies like voice recognition and machine learning can analyze patient journals and possibly, in the future, social media feeds to detect mental health issues. These technologies aim to extend and augment the capabilities of healthcare practices, improving the identification and management of patients at risk for mental health issues.
Q: Are these technologies as effective in pediatric populations, and are there any specific challenges?
A: Technologies for mental health screening and support are effective in pediatric populations, with certain age-specific considerations and legal restrictions on technology use. For adolescents and older children comfortable with technology, digital tools can significantly impact mental health care. For younger children, technology must facilitate information-gathering from various sources, including parents and teachers. Despite challenges, technology is crucial for early identification and intervention in pediatric mental health, potentially shortening the time to diagnosis and improving outcomes.
The statistics are horrifying. One third of adolescent girls have seriously thought about suicide over the past year; 13% attempt suicide. So there’s a need in the adolescent population and in the preadolescent population, too, because there’s an 8- to 10-year lag between onset of symptoms and diagnosis of mental illness. If we can shorten that lag, you see improved performance in schools; you see decreased truancy; you see greater economic achievement and so on. It makes such a profound difference. Not to mention it saves lives. So, yes, technology is critical in a pediatric population. It exists and it’s happening right now. There are challenges, but the goal can be met.
Q: A 2014 study found that 45% of people who completed suicide visited a primary care physician in the preceding month. And only 23% of people who attempt suicide have not seen a primary care physician within the past year. What does that say about the importance of screening at the primary care level?
A: The fact that a significant percentage of individuals who die by suicide have visited a primary care physician within a month or year prior to their death underscores the critical role of primary care in suicide prevention. This highlights the potential for primary care settings to identify and intervene with individuals at risk for suicide, making the case for the importance of integrating effective mental health screenings and support technologies in primary care practices.
Q: In other words, we’re not talking about a marginal benefit.
A: No, the potential benefit is huge. The United States Preventive Services Task Force did not endorse universal screening for suicide in its 2023 recommendations; they felt — and I accept that conclusion — there wasn›t enough evidence [at the time] to really support that recommendation. I think when you talk to a lot of suicide researchers, what you will hear is that providing suicide assessments as far upstream as possible is critical, especially when you start seeing more and more research showing that 20% of the population who die by suicide are not likely to have any psychiatric pathology at all. I believe the evidence base will soon support a recommendation for universal screening for adults. I believe it is especially important to screen for suicidal ideation in kids, given the high rates of suicide in this population.
Dr. Zaubler has disclosed the following relevant financial relationships: chief medical officer, NeuroFlow.
A version of this article appeared on Medscape.com.
Primary care physicians play a critical role in identifying patients at risk for serious mental health issues, including suicidality. But the ever-increasing demands on their clinical time can hinder the ability to identify emotional distress in time to intervene. Can artificial intelligence (AI) help?
This news organization spoke with Tom Zaubler, MD, a psychiatrist and chief medical officer of NeuroFlow, about how AI can improve the ability of primary care physicians and other clinicians to screen their patients for suicidal ideation and boost rates of treatment for mental health issues in their patients. This interview has been edited for clarity and length.
Question: How can AI help in suicide prevention and mental health screening in primary care?
Answer: Recent studies have demonstrated the potential of AI in mental health screening and suicide prevention. One method is natural language processing (NLP), which can analyze patients› journal entries for signs of suicidal thoughts or behaviors. This technology has shown promise in detecting suicidal ideation in patients who may not report such thoughts on traditional screening tools like the Patient Health Questionnaire-9 (PHQ-9). AI can be part of an integrated approach to identify and provide support to individuals at risk for suicide or those without a psychiatric history but who may still be at risk.
Q: A recent study by [Maria] Oquendo and colleagues found that one fifth of patients who attempt suicide do not meet the criteria for a mental health disorder.
Improved screening is obviously important, but in some ways it’s not the most important part of the problem. The lack of accessibility to specialized mental health care is a critical obstacle to treating patients with acute psychiatric needs.
How can primary care doctors effectively connect patients with mental health support, given the scarcity of mental health professionals?
A: Primary care doctors can leverage technology to extend mental health support. This includes using platforms for safety screening and providing patients with immediate access to local and national resources and digital interventions. Alerts can be sent to professionals within the practice or employed by technology companies to offer immediate support, including suicide safety planning and counseling. Users can hit a button to “Find a Therapist.” Also, if they acknowledge feelings of self-harm, these keywords are detected within the app by NLP. “Urgent alerts” are then sent to clinicians who are overseeing patient care. If someone is flagged, a social worker or member of a response services team intervenes and calls the person at risk to tailor care. These interventions do not always require a psychiatrist or masters-prepared clinician but can be effectively managed by trained paraprofessionals. These staff members can provide suicide safety planning and lethal-means-restriction counseling, and can assess the need for escalation of care.
Q: How is technology likely to manifest in physician practices in the near future to support mental health care?
A: Automated screening platforms for depression and anxiety, alerts for physicians when patients screen positively, and integration with collaborative care models are a few of the ways technology will become part of clinical practice. Additionally, advanced data analytics and predictive modeling using electronic health records and claims data will help identify high-risk patients. Technologies like voice recognition and machine learning can analyze patient journals and possibly, in the future, social media feeds to detect mental health issues. These technologies aim to extend and augment the capabilities of healthcare practices, improving the identification and management of patients at risk for mental health issues.
Q: Are these technologies as effective in pediatric populations, and are there any specific challenges?
A: Technologies for mental health screening and support are effective in pediatric populations, with certain age-specific considerations and legal restrictions on technology use. For adolescents and older children comfortable with technology, digital tools can significantly impact mental health care. For younger children, technology must facilitate information-gathering from various sources, including parents and teachers. Despite challenges, technology is crucial for early identification and intervention in pediatric mental health, potentially shortening the time to diagnosis and improving outcomes.
The statistics are horrifying. One third of adolescent girls have seriously thought about suicide over the past year; 13% attempt suicide. So there’s a need in the adolescent population and in the preadolescent population, too, because there’s an 8- to 10-year lag between onset of symptoms and diagnosis of mental illness. If we can shorten that lag, you see improved performance in schools; you see decreased truancy; you see greater economic achievement and so on. It makes such a profound difference. Not to mention it saves lives. So, yes, technology is critical in a pediatric population. It exists and it’s happening right now. There are challenges, but the goal can be met.
Q: A 2014 study found that 45% of people who completed suicide visited a primary care physician in the preceding month. And only 23% of people who attempt suicide have not seen a primary care physician within the past year. What does that say about the importance of screening at the primary care level?
A: The fact that a significant percentage of individuals who die by suicide have visited a primary care physician within a month or year prior to their death underscores the critical role of primary care in suicide prevention. This highlights the potential for primary care settings to identify and intervene with individuals at risk for suicide, making the case for the importance of integrating effective mental health screenings and support technologies in primary care practices.
Q: In other words, we’re not talking about a marginal benefit.
A: No, the potential benefit is huge. The United States Preventive Services Task Force did not endorse universal screening for suicide in its 2023 recommendations; they felt — and I accept that conclusion — there wasn›t enough evidence [at the time] to really support that recommendation. I think when you talk to a lot of suicide researchers, what you will hear is that providing suicide assessments as far upstream as possible is critical, especially when you start seeing more and more research showing that 20% of the population who die by suicide are not likely to have any psychiatric pathology at all. I believe the evidence base will soon support a recommendation for universal screening for adults. I believe it is especially important to screen for suicidal ideation in kids, given the high rates of suicide in this population.
Dr. Zaubler has disclosed the following relevant financial relationships: chief medical officer, NeuroFlow.
A version of this article appeared on Medscape.com.
Florida Legislature Passes Free Skin Cancer Screening Requirement
By this summer, state employees in Florida covered by state group health insurance plans should have access to free annual skin cancer screenings.
On March 1, 2024, legislation was unanimously passed by both chambers of the state legislature that will provide for the free screenings for this group as of July 1. Some 321,000 state employees would be eligible, at a cost of about $357,000 per year, according to a legislative analysis. Gov. Ron DeSantis (R) has received and is expected to sign the bill.
The analysis concluded that the bill would have a “significant negative fiscal impact on the state employee group health plan,” as screenings will ultimately reduce cancer incidence and related morbidity and mortality.
The screenings aim to provide access to patients who may think they might not be able to afford a visit or who may have other perceived or real barriers to going for a skin check, said Sima Jain, MD, president of the Florida Academy of Dermatology. “It’s really meant to give patients access who need it,” said Dr. Jain, a dermatologist in private practice in Orlando.
The goal is early detection. “If I do a simple excision on a melanoma and we catch it early, it’s done, it’s cured,” Dr. Jain told this news organization. “It’s a win-win. We catch it early and insurance companies pay less money,” she said.
An effort to have all insurers in the state provide free screenings failed in 2023.
From 2016 to 2020, Florida had a higher overall incidence of melanoma at 25.4 per 100,000 than the national average of 22.5, according to the National Cancer Institute. The state had some 7500 cases of melanoma each year during that period. The incidence rate in some Florida counties is as high as 32.7-45.6 per 100,000.
The Florida legislation will allow physician assistants and advanced practice nurses who operate under the supervision of a dermatologist to conduct the screenings.
It’s not clear how many state employees will access the free skin checks. “I don’t expect to see a flood of skin cancer screenings,” said Dr. Jain, noting that she hopes that it attracts primarily those at highest risk.
Once the bill is signed by the governor, Florida will be the second state to cover skin cancer screenings in some way. Illinois has required free skin cancer screening for all insured residents since 2020.
A version of this article appeared on Medscape.com .
By this summer, state employees in Florida covered by state group health insurance plans should have access to free annual skin cancer screenings.
On March 1, 2024, legislation was unanimously passed by both chambers of the state legislature that will provide for the free screenings for this group as of July 1. Some 321,000 state employees would be eligible, at a cost of about $357,000 per year, according to a legislative analysis. Gov. Ron DeSantis (R) has received and is expected to sign the bill.
The analysis concluded that the bill would have a “significant negative fiscal impact on the state employee group health plan,” as screenings will ultimately reduce cancer incidence and related morbidity and mortality.
The screenings aim to provide access to patients who may think they might not be able to afford a visit or who may have other perceived or real barriers to going for a skin check, said Sima Jain, MD, president of the Florida Academy of Dermatology. “It’s really meant to give patients access who need it,” said Dr. Jain, a dermatologist in private practice in Orlando.
The goal is early detection. “If I do a simple excision on a melanoma and we catch it early, it’s done, it’s cured,” Dr. Jain told this news organization. “It’s a win-win. We catch it early and insurance companies pay less money,” she said.
An effort to have all insurers in the state provide free screenings failed in 2023.
From 2016 to 2020, Florida had a higher overall incidence of melanoma at 25.4 per 100,000 than the national average of 22.5, according to the National Cancer Institute. The state had some 7500 cases of melanoma each year during that period. The incidence rate in some Florida counties is as high as 32.7-45.6 per 100,000.
The Florida legislation will allow physician assistants and advanced practice nurses who operate under the supervision of a dermatologist to conduct the screenings.
It’s not clear how many state employees will access the free skin checks. “I don’t expect to see a flood of skin cancer screenings,” said Dr. Jain, noting that she hopes that it attracts primarily those at highest risk.
Once the bill is signed by the governor, Florida will be the second state to cover skin cancer screenings in some way. Illinois has required free skin cancer screening for all insured residents since 2020.
A version of this article appeared on Medscape.com .
By this summer, state employees in Florida covered by state group health insurance plans should have access to free annual skin cancer screenings.
On March 1, 2024, legislation was unanimously passed by both chambers of the state legislature that will provide for the free screenings for this group as of July 1. Some 321,000 state employees would be eligible, at a cost of about $357,000 per year, according to a legislative analysis. Gov. Ron DeSantis (R) has received and is expected to sign the bill.
The analysis concluded that the bill would have a “significant negative fiscal impact on the state employee group health plan,” as screenings will ultimately reduce cancer incidence and related morbidity and mortality.
The screenings aim to provide access to patients who may think they might not be able to afford a visit or who may have other perceived or real barriers to going for a skin check, said Sima Jain, MD, president of the Florida Academy of Dermatology. “It’s really meant to give patients access who need it,” said Dr. Jain, a dermatologist in private practice in Orlando.
The goal is early detection. “If I do a simple excision on a melanoma and we catch it early, it’s done, it’s cured,” Dr. Jain told this news organization. “It’s a win-win. We catch it early and insurance companies pay less money,” she said.
An effort to have all insurers in the state provide free screenings failed in 2023.
From 2016 to 2020, Florida had a higher overall incidence of melanoma at 25.4 per 100,000 than the national average of 22.5, according to the National Cancer Institute. The state had some 7500 cases of melanoma each year during that period. The incidence rate in some Florida counties is as high as 32.7-45.6 per 100,000.
The Florida legislation will allow physician assistants and advanced practice nurses who operate under the supervision of a dermatologist to conduct the screenings.
It’s not clear how many state employees will access the free skin checks. “I don’t expect to see a flood of skin cancer screenings,” said Dr. Jain, noting that she hopes that it attracts primarily those at highest risk.
Once the bill is signed by the governor, Florida will be the second state to cover skin cancer screenings in some way. Illinois has required free skin cancer screening for all insured residents since 2020.
A version of this article appeared on Medscape.com .
Commentary: Gut Dysbiosis, DMARD, Joint Involvement, and MACE in PsA, April 2024
After PsA onset, early diagnosis and management leads to better long-term outcomes. These prior observations were confirmed in a study by Snoeck Henkemans and colleagues that included 708 newly diagnosed patients with PsA naive to disease-modifying antirheumatic drugs (DMARD) who were followed up for 3 years or more. Patients with a short (<12 weeks) vs long delay (>1 year) in PsA diagnosis after symptom onset were more likely to achieve minimum disease activity (OR 2.55; 95% CI 1.37-4.76). Thus, longer delay in diagnosing PsA is associated with worse clinical outcomes.
Bimekizumab is a novel biologic therapy that inhibits interleukins (IL)-17A and -17F and is efficacious in the treatment of psoriasis, PsA, and axial spondyloarthritis. However, the effectiveness in PsA vis-à-vis other IL-17A inhibitors is not known. In the absence of a formal head-to-head study, matching-adjusted indirect comparisons is a method to evaluate comparative effectiveness. Such a study by Mease and colleagues included the data of patients with PsA who were biological DMARD–naive or who had an inadequate response to tumor necrosis factor inhibitors (TNFi-IR), and who received bimekizumab from the BE OPTIMAL (n = 236) and BE COMPLETE (n = 146) trials and secukinumab from the FUTURE 2 trial (n = 200). They demonstrated that, in the biological DMARD–naive subgroup, the probability of achieving at least 70% improvement in American College of Rheumatology (ACR) response was two times higher with bimekizumab (160 mg every 4 weeks) vs secukinumab (150 mg or 300 mg every 4 weeks) at week 52. In the TNFi-IR subgroup, bimekizumab had a greater likelihood of response compared with 150 mg secukinumab for ACR20, ACR70, and minimal disease activity outcomes and a greater likelihood of response compared with 300 mg secukinumab for ACR50 and minimal disease activity. Thus, bimekizumab is at least as effective as secukinumab in PsA. Formal head-to-head studies comparing bimekizumab with other IL-17A inhibitors are required.
Distal interphalangeal (DIP) joint involvement is an important manifestation of PsA and is closely related to nail dystrophy in the adjacent nail. Ixekizumab is another biologic that targets IL-17A. In a post hoc analysis of the SPIRIT-H2H study, McGonagle and colleagues confirmed that over 96% of patients with PsA and simultaneous DIP joint involvement reported adjacent nail psoriasis. When compared with adalimumab, ixekizumab led to greater improvements in DIP involvement and adjacent nail psoriasis as early as week 12 (38.8% vs 28.4%; P < .0001), with improvements sustained up to week 52 (64.9% vs 57.5%; P = .0055). This probably reflects a greater effectiveness of IL-17A inhibition in treating skin and nail psoriasis compared with TNFi.
Finally, in a population-based retrospective cohort study that included 13,905 patients with PsA (n = 1672) or rheumatoid arthritis (n = 12,233) who did not have any previous history of major adverse cardiovascular events (MACE), Meng and colleagues showed that the incidence rates of MACE were similar in patients with PsA and rheumatoid arthritis. Thus, cardiovascular risk management should be similarly aggressive in patients with PsA and rheumatoid arthritis.
After PsA onset, early diagnosis and management leads to better long-term outcomes. These prior observations were confirmed in a study by Snoeck Henkemans and colleagues that included 708 newly diagnosed patients with PsA naive to disease-modifying antirheumatic drugs (DMARD) who were followed up for 3 years or more. Patients with a short (<12 weeks) vs long delay (>1 year) in PsA diagnosis after symptom onset were more likely to achieve minimum disease activity (OR 2.55; 95% CI 1.37-4.76). Thus, longer delay in diagnosing PsA is associated with worse clinical outcomes.
Bimekizumab is a novel biologic therapy that inhibits interleukins (IL)-17A and -17F and is efficacious in the treatment of psoriasis, PsA, and axial spondyloarthritis. However, the effectiveness in PsA vis-à-vis other IL-17A inhibitors is not known. In the absence of a formal head-to-head study, matching-adjusted indirect comparisons is a method to evaluate comparative effectiveness. Such a study by Mease and colleagues included the data of patients with PsA who were biological DMARD–naive or who had an inadequate response to tumor necrosis factor inhibitors (TNFi-IR), and who received bimekizumab from the BE OPTIMAL (n = 236) and BE COMPLETE (n = 146) trials and secukinumab from the FUTURE 2 trial (n = 200). They demonstrated that, in the biological DMARD–naive subgroup, the probability of achieving at least 70% improvement in American College of Rheumatology (ACR) response was two times higher with bimekizumab (160 mg every 4 weeks) vs secukinumab (150 mg or 300 mg every 4 weeks) at week 52. In the TNFi-IR subgroup, bimekizumab had a greater likelihood of response compared with 150 mg secukinumab for ACR20, ACR70, and minimal disease activity outcomes and a greater likelihood of response compared with 300 mg secukinumab for ACR50 and minimal disease activity. Thus, bimekizumab is at least as effective as secukinumab in PsA. Formal head-to-head studies comparing bimekizumab with other IL-17A inhibitors are required.
Distal interphalangeal (DIP) joint involvement is an important manifestation of PsA and is closely related to nail dystrophy in the adjacent nail. Ixekizumab is another biologic that targets IL-17A. In a post hoc analysis of the SPIRIT-H2H study, McGonagle and colleagues confirmed that over 96% of patients with PsA and simultaneous DIP joint involvement reported adjacent nail psoriasis. When compared with adalimumab, ixekizumab led to greater improvements in DIP involvement and adjacent nail psoriasis as early as week 12 (38.8% vs 28.4%; P < .0001), with improvements sustained up to week 52 (64.9% vs 57.5%; P = .0055). This probably reflects a greater effectiveness of IL-17A inhibition in treating skin and nail psoriasis compared with TNFi.
Finally, in a population-based retrospective cohort study that included 13,905 patients with PsA (n = 1672) or rheumatoid arthritis (n = 12,233) who did not have any previous history of major adverse cardiovascular events (MACE), Meng and colleagues showed that the incidence rates of MACE were similar in patients with PsA and rheumatoid arthritis. Thus, cardiovascular risk management should be similarly aggressive in patients with PsA and rheumatoid arthritis.
After PsA onset, early diagnosis and management leads to better long-term outcomes. These prior observations were confirmed in a study by Snoeck Henkemans and colleagues that included 708 newly diagnosed patients with PsA naive to disease-modifying antirheumatic drugs (DMARD) who were followed up for 3 years or more. Patients with a short (<12 weeks) vs long delay (>1 year) in PsA diagnosis after symptom onset were more likely to achieve minimum disease activity (OR 2.55; 95% CI 1.37-4.76). Thus, longer delay in diagnosing PsA is associated with worse clinical outcomes.
Bimekizumab is a novel biologic therapy that inhibits interleukins (IL)-17A and -17F and is efficacious in the treatment of psoriasis, PsA, and axial spondyloarthritis. However, the effectiveness in PsA vis-à-vis other IL-17A inhibitors is not known. In the absence of a formal head-to-head study, matching-adjusted indirect comparisons is a method to evaluate comparative effectiveness. Such a study by Mease and colleagues included the data of patients with PsA who were biological DMARD–naive or who had an inadequate response to tumor necrosis factor inhibitors (TNFi-IR), and who received bimekizumab from the BE OPTIMAL (n = 236) and BE COMPLETE (n = 146) trials and secukinumab from the FUTURE 2 trial (n = 200). They demonstrated that, in the biological DMARD–naive subgroup, the probability of achieving at least 70% improvement in American College of Rheumatology (ACR) response was two times higher with bimekizumab (160 mg every 4 weeks) vs secukinumab (150 mg or 300 mg every 4 weeks) at week 52. In the TNFi-IR subgroup, bimekizumab had a greater likelihood of response compared with 150 mg secukinumab for ACR20, ACR70, and minimal disease activity outcomes and a greater likelihood of response compared with 300 mg secukinumab for ACR50 and minimal disease activity. Thus, bimekizumab is at least as effective as secukinumab in PsA. Formal head-to-head studies comparing bimekizumab with other IL-17A inhibitors are required.
Distal interphalangeal (DIP) joint involvement is an important manifestation of PsA and is closely related to nail dystrophy in the adjacent nail. Ixekizumab is another biologic that targets IL-17A. In a post hoc analysis of the SPIRIT-H2H study, McGonagle and colleagues confirmed that over 96% of patients with PsA and simultaneous DIP joint involvement reported adjacent nail psoriasis. When compared with adalimumab, ixekizumab led to greater improvements in DIP involvement and adjacent nail psoriasis as early as week 12 (38.8% vs 28.4%; P < .0001), with improvements sustained up to week 52 (64.9% vs 57.5%; P = .0055). This probably reflects a greater effectiveness of IL-17A inhibition in treating skin and nail psoriasis compared with TNFi.
Finally, in a population-based retrospective cohort study that included 13,905 patients with PsA (n = 1672) or rheumatoid arthritis (n = 12,233) who did not have any previous history of major adverse cardiovascular events (MACE), Meng and colleagues showed that the incidence rates of MACE were similar in patients with PsA and rheumatoid arthritis. Thus, cardiovascular risk management should be similarly aggressive in patients with PsA and rheumatoid arthritis.
Commentary: MRI Surveillance and Risk Factors in Breast Cancer, April 2024
A positive family history of cancer and obesity are established risk factors for development of breast cancer among women.[4,5] A population-based cohort study that included 15,055 Chinese women evaluated the association and interaction between body mass index (BMI) and family history of cancer on the risk for breast cancer (Cao et al). The incidence risk for breast cancer was highest in the group with obesity vs the group with normal weight (adjusted HR 2.09; 95% CI 1.42-3.07), and those with a family history of cancer also had an increased risk vs those without a family history of cancer (adjusted HR 1.63; 95% CI 1.22-2.49). Furthermore, women with a BMI ≥ 24 and family history of cancer had a higher risk for breast cancer development compared with women with a BMI < 24 and no family history of cancer (adjusted HR 2.06; 95% CI 1.39-3.06). This study indicates a heightened breast cancer risk when cancer family history and obesity coexist, suggesting the importance of addressing modifiable risk factors and targeting lifestyle interventions in this population.
Triple-negative breast cancer (TNBC), although exhibiting its own heterogeneity, has various features that differentiate this subtype from luminal breast cancers. For example, TNBC generally has a more aggressive course, increased responsiveness to chemotherapy, and earlier pattern of recurrence compared with hormone receptor–positive disease. Prior studies have also shown that established breast cancer risk factors reflect those for the luminal A subtype, whereas those for TNBC are less consistent.[6] A meta-analysis that included 33 studies evaluated the association between traditional breast cancer risk factors and TNBC incidence (Kumar et al). Family history (odds ratio [OR] 1.55; 95% CI 1.34-1.81; P < .001), longer duration of oral contraceptive use (OR 1.29; 95% CI 1.08-1.55; P < .001), and higher breast density (OR 2.19; 95% CI 1.67-2.88; P < .001) were significantly associated with an increased risk for TNBC. Factors including later age at menarche, later age at first birth, and breastfeeding were associated with reduced risk for TNBC. Furthermore, there was no significant association with parity, menopausal hormone therapy, alcohol, smoking, and BMI. This study highlights distinct risk factors that may contribute to a higher risk for TNBC, and future research will be valuable to better elucidate the mechanisms at play and to further understand the differences within this subtype itself.
Additional References
- National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology. Genetic/familial high-risk assessment: breast, ovarian, and pancreatic. Version 3.2024. Source
- Saadatmand S, Geuzinge HA, Rutgers EJT, et al; on behalf of the FaMRIsc study group. MRI versus mammography for breast cancer screening in women with familial risk (FaMRIsc): A multicentre, randomised, controlled trial. Lancet Oncol. 2019;20:1136-1147. doi: 10.1016/S1470-2045(19)30275-X Source
- Warner E, Zhu S, Plewes DB, et al. Breast cancer mortality among women with a BRCA1 or BRCA2 mutation in a magnetic resonance imaging plus mammography screening program. Cancers (Basel). 2020;12:3479. doi: 10.3390/cancers12113479 Source
- Picon-Ruiz M, Morata-Tarifa C, Valle-Goffin JJ, et al. Obesity and adverse breast cancer risk and outcome: Mechanistic insights and strategies for intervention. CA Cancer J Clin. 2017;67:378-397. doi: 10.3322/caac.21405 Source
- Engmann NJ, Golmakani MK, Miglioretti DL, et al; for the Breast Cancer Surveillance Consortium. Population-attributable risk proportion of clinical risk factors for breast cancer. JAMA Oncol. 2017;3:1228-1236. doi: 10.1001/jamaoncol.2016.6326 Source
- Barnard ME, Boeke CE, Tamimi RM. Established breast cancer risk factors and risk of intrinsic tumor subtypes. Biochim Biophys Acta Rev Cancer. 2015;1856:73-85. doi: 10.1016/j.bbcan.2015.0002 Source
A positive family history of cancer and obesity are established risk factors for development of breast cancer among women.[4,5] A population-based cohort study that included 15,055 Chinese women evaluated the association and interaction between body mass index (BMI) and family history of cancer on the risk for breast cancer (Cao et al). The incidence risk for breast cancer was highest in the group with obesity vs the group with normal weight (adjusted HR 2.09; 95% CI 1.42-3.07), and those with a family history of cancer also had an increased risk vs those without a family history of cancer (adjusted HR 1.63; 95% CI 1.22-2.49). Furthermore, women with a BMI ≥ 24 and family history of cancer had a higher risk for breast cancer development compared with women with a BMI < 24 and no family history of cancer (adjusted HR 2.06; 95% CI 1.39-3.06). This study indicates a heightened breast cancer risk when cancer family history and obesity coexist, suggesting the importance of addressing modifiable risk factors and targeting lifestyle interventions in this population.
Triple-negative breast cancer (TNBC), although exhibiting its own heterogeneity, has various features that differentiate this subtype from luminal breast cancers. For example, TNBC generally has a more aggressive course, increased responsiveness to chemotherapy, and earlier pattern of recurrence compared with hormone receptor–positive disease. Prior studies have also shown that established breast cancer risk factors reflect those for the luminal A subtype, whereas those for TNBC are less consistent.[6] A meta-analysis that included 33 studies evaluated the association between traditional breast cancer risk factors and TNBC incidence (Kumar et al). Family history (odds ratio [OR] 1.55; 95% CI 1.34-1.81; P < .001), longer duration of oral contraceptive use (OR 1.29; 95% CI 1.08-1.55; P < .001), and higher breast density (OR 2.19; 95% CI 1.67-2.88; P < .001) were significantly associated with an increased risk for TNBC. Factors including later age at menarche, later age at first birth, and breastfeeding were associated with reduced risk for TNBC. Furthermore, there was no significant association with parity, menopausal hormone therapy, alcohol, smoking, and BMI. This study highlights distinct risk factors that may contribute to a higher risk for TNBC, and future research will be valuable to better elucidate the mechanisms at play and to further understand the differences within this subtype itself.
Additional References
- National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology. Genetic/familial high-risk assessment: breast, ovarian, and pancreatic. Version 3.2024. Source
- Saadatmand S, Geuzinge HA, Rutgers EJT, et al; on behalf of the FaMRIsc study group. MRI versus mammography for breast cancer screening in women with familial risk (FaMRIsc): A multicentre, randomised, controlled trial. Lancet Oncol. 2019;20:1136-1147. doi: 10.1016/S1470-2045(19)30275-X Source
- Warner E, Zhu S, Plewes DB, et al. Breast cancer mortality among women with a BRCA1 or BRCA2 mutation in a magnetic resonance imaging plus mammography screening program. Cancers (Basel). 2020;12:3479. doi: 10.3390/cancers12113479 Source
- Picon-Ruiz M, Morata-Tarifa C, Valle-Goffin JJ, et al. Obesity and adverse breast cancer risk and outcome: Mechanistic insights and strategies for intervention. CA Cancer J Clin. 2017;67:378-397. doi: 10.3322/caac.21405 Source
- Engmann NJ, Golmakani MK, Miglioretti DL, et al; for the Breast Cancer Surveillance Consortium. Population-attributable risk proportion of clinical risk factors for breast cancer. JAMA Oncol. 2017;3:1228-1236. doi: 10.1001/jamaoncol.2016.6326 Source
- Barnard ME, Boeke CE, Tamimi RM. Established breast cancer risk factors and risk of intrinsic tumor subtypes. Biochim Biophys Acta Rev Cancer. 2015;1856:73-85. doi: 10.1016/j.bbcan.2015.0002 Source
A positive family history of cancer and obesity are established risk factors for development of breast cancer among women.[4,5] A population-based cohort study that included 15,055 Chinese women evaluated the association and interaction between body mass index (BMI) and family history of cancer on the risk for breast cancer (Cao et al). The incidence risk for breast cancer was highest in the group with obesity vs the group with normal weight (adjusted HR 2.09; 95% CI 1.42-3.07), and those with a family history of cancer also had an increased risk vs those without a family history of cancer (adjusted HR 1.63; 95% CI 1.22-2.49). Furthermore, women with a BMI ≥ 24 and family history of cancer had a higher risk for breast cancer development compared with women with a BMI < 24 and no family history of cancer (adjusted HR 2.06; 95% CI 1.39-3.06). This study indicates a heightened breast cancer risk when cancer family history and obesity coexist, suggesting the importance of addressing modifiable risk factors and targeting lifestyle interventions in this population.
Triple-negative breast cancer (TNBC), although exhibiting its own heterogeneity, has various features that differentiate this subtype from luminal breast cancers. For example, TNBC generally has a more aggressive course, increased responsiveness to chemotherapy, and earlier pattern of recurrence compared with hormone receptor–positive disease. Prior studies have also shown that established breast cancer risk factors reflect those for the luminal A subtype, whereas those for TNBC are less consistent.[6] A meta-analysis that included 33 studies evaluated the association between traditional breast cancer risk factors and TNBC incidence (Kumar et al). Family history (odds ratio [OR] 1.55; 95% CI 1.34-1.81; P < .001), longer duration of oral contraceptive use (OR 1.29; 95% CI 1.08-1.55; P < .001), and higher breast density (OR 2.19; 95% CI 1.67-2.88; P < .001) were significantly associated with an increased risk for TNBC. Factors including later age at menarche, later age at first birth, and breastfeeding were associated with reduced risk for TNBC. Furthermore, there was no significant association with parity, menopausal hormone therapy, alcohol, smoking, and BMI. This study highlights distinct risk factors that may contribute to a higher risk for TNBC, and future research will be valuable to better elucidate the mechanisms at play and to further understand the differences within this subtype itself.
Additional References
- National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology. Genetic/familial high-risk assessment: breast, ovarian, and pancreatic. Version 3.2024. Source
- Saadatmand S, Geuzinge HA, Rutgers EJT, et al; on behalf of the FaMRIsc study group. MRI versus mammography for breast cancer screening in women with familial risk (FaMRIsc): A multicentre, randomised, controlled trial. Lancet Oncol. 2019;20:1136-1147. doi: 10.1016/S1470-2045(19)30275-X Source
- Warner E, Zhu S, Plewes DB, et al. Breast cancer mortality among women with a BRCA1 or BRCA2 mutation in a magnetic resonance imaging plus mammography screening program. Cancers (Basel). 2020;12:3479. doi: 10.3390/cancers12113479 Source
- Picon-Ruiz M, Morata-Tarifa C, Valle-Goffin JJ, et al. Obesity and adverse breast cancer risk and outcome: Mechanistic insights and strategies for intervention. CA Cancer J Clin. 2017;67:378-397. doi: 10.3322/caac.21405 Source
- Engmann NJ, Golmakani MK, Miglioretti DL, et al; for the Breast Cancer Surveillance Consortium. Population-attributable risk proportion of clinical risk factors for breast cancer. JAMA Oncol. 2017;3:1228-1236. doi: 10.1001/jamaoncol.2016.6326 Source
- Barnard ME, Boeke CE, Tamimi RM. Established breast cancer risk factors and risk of intrinsic tumor subtypes. Biochim Biophys Acta Rev Cancer. 2015;1856:73-85. doi: 10.1016/j.bbcan.2015.0002 Source
Tarlatamab Shows Promise in Tackling Previously Treated SCLC
The investigational bispecific T-cell engager tarlatamab achieved durable responses and clinically meaningful survival outcomes in patients with small-cell lung cancer (SCLC), particularly at lower doses, according to a follow-up analysis of the phase 1 DeLLphi-300 trial.
Most patients with central nervous system tumors also sustained tumor shrinkage long after receiving radiotherapy, providing “encouraging evidence” of the new agent’s intracranial activity, said study presenter Horst-Dieter Hummel, MD, Comprehensive Cancer Center Mainfranken, Würzburg, Germany.
The research was presented at the European Lung Cancer Congress 2024 on March 22.
Data from the phase 1 and phase 2 DeLLphi trials, published last year, showed the compound achieved “encouraging clinical activity” in pretreated patients, said Dr. Hummel.
The initial phase 1 DeLLphi study found that after a median follow-up of 8.7 months, the immunotherapy led to a disease control rate of 51.4%, a median progression-free survival of 3.7 months, and median overall survival of 13.2 months.
At the meeting, Dr. Hummel reported longer-term outcomes from the phase 1 study over a median of 12.1 months as well as intracranial activity in patients who received clinically relevant doses of tarlatamab, defined as ≥ 10 mg.
The 152 patients included in the analysis had a median of two prior lines of therapy; 76.3% had undergone radiotherapy, and 63.2% had received immunotherapy. Liver metastases were present in 42.1% of patients, and 25.0% had brain metastases.
Doses varied among participants, with 76 patients (50.0%) receiving 100 mg, 32 (21.0%) receiving 100 mg via extended intravenous infusion, 17 (11.2%) receiving 10 mg, and 8 (5.3%) receiving 30 mg.
The overall objective response rate was 25.0%, with a median duration of response of 11.2 months. Among patients given the 10-mg dose, the objective response rate was higher, at 35.3%, as was the median duration of response, at 14.9 months.
Tarlatamab was associated with a median overall survival of 17.5 months, with 57.9% of patients alive at 12 months. Patients receiving the 10 mg dose had a better median overall survival of 20.3 months.
Of the 16 patients with analyzable central nervous system tumors, 62.5% experienced tumor shrinkage by ≥ 30% and 87.5% experienced intracranial disease control, which lasted for a median of 7.4 months.
In this follow-up study, tarlatamab demonstrated “clinically meaningful survival outcomes in patients with previously treated SCLC, particularly with the 10 mg dose,” Dr. Hummel concluded in his presentation.
No new safety signals emerged, though almost all patients did experience tarlatamab-related adverse events (94.8% for doses > 10 mg and 100% of patients with 10 mg doses). Overall, 66.4% of the total cohort experienced cytokine release syndrome of any grade, and 11.8% developed immune effector cell-associated neurotoxicity syndrome.
Discontinuation due to treatment-related adverse events occurred in 9 patients overall, and adverse events that led to dose interruption or reduction occurred in 32 patients overall.
“After many efforts at DLL3 targeting, we finally have an agent that shows activity and efficacy, and with convincing data,” said Jessica Menis, MD, a medical oncologist at the oncology department of the University Hospital of Verona, Italy, who was not involved in the study. The intracranial activity of tarlatamab “needs to be further evaluated in untreated patients,” Dr. Menis noted, because the study included only patients with stable, treated brain metastases.
And given the high rates of adverse events, Dr. Menis cautioned that adverse event management “will be a challenge.”
On X (Twitter), Tom Newsom-Davis, MBBS, PhD, a consultant in medical oncology at Chelsea and Westminster Hospital, London, said that tarlatamab is “not a straightforward drug to use,” highlighting the occurrence of cytokine release syndrome.
“But in this significantly pretreated population and in this hard-to-treat tumor type,” the rate and duration of responses seen with the extended follow-up are ‘impressive’,” he added.
DeLLphi-300, 301, and 304 were funded by Amgen Inc. Dr. Hummel declared relationships with several companies, including Amgen, Bristol Myers Squibb, AstraZeneca, Celgene, Merck, Novartis, Daiichi Sankyo, and Roche. Dr. Menis declared relationships with AstraZeneca, BMS, MSD, Roche, and Novartis.
A version of this article appeared on Medscape.com.
The investigational bispecific T-cell engager tarlatamab achieved durable responses and clinically meaningful survival outcomes in patients with small-cell lung cancer (SCLC), particularly at lower doses, according to a follow-up analysis of the phase 1 DeLLphi-300 trial.
Most patients with central nervous system tumors also sustained tumor shrinkage long after receiving radiotherapy, providing “encouraging evidence” of the new agent’s intracranial activity, said study presenter Horst-Dieter Hummel, MD, Comprehensive Cancer Center Mainfranken, Würzburg, Germany.
The research was presented at the European Lung Cancer Congress 2024 on March 22.
Data from the phase 1 and phase 2 DeLLphi trials, published last year, showed the compound achieved “encouraging clinical activity” in pretreated patients, said Dr. Hummel.
The initial phase 1 DeLLphi study found that after a median follow-up of 8.7 months, the immunotherapy led to a disease control rate of 51.4%, a median progression-free survival of 3.7 months, and median overall survival of 13.2 months.
At the meeting, Dr. Hummel reported longer-term outcomes from the phase 1 study over a median of 12.1 months as well as intracranial activity in patients who received clinically relevant doses of tarlatamab, defined as ≥ 10 mg.
The 152 patients included in the analysis had a median of two prior lines of therapy; 76.3% had undergone radiotherapy, and 63.2% had received immunotherapy. Liver metastases were present in 42.1% of patients, and 25.0% had brain metastases.
Doses varied among participants, with 76 patients (50.0%) receiving 100 mg, 32 (21.0%) receiving 100 mg via extended intravenous infusion, 17 (11.2%) receiving 10 mg, and 8 (5.3%) receiving 30 mg.
The overall objective response rate was 25.0%, with a median duration of response of 11.2 months. Among patients given the 10-mg dose, the objective response rate was higher, at 35.3%, as was the median duration of response, at 14.9 months.
Tarlatamab was associated with a median overall survival of 17.5 months, with 57.9% of patients alive at 12 months. Patients receiving the 10 mg dose had a better median overall survival of 20.3 months.
Of the 16 patients with analyzable central nervous system tumors, 62.5% experienced tumor shrinkage by ≥ 30% and 87.5% experienced intracranial disease control, which lasted for a median of 7.4 months.
In this follow-up study, tarlatamab demonstrated “clinically meaningful survival outcomes in patients with previously treated SCLC, particularly with the 10 mg dose,” Dr. Hummel concluded in his presentation.
No new safety signals emerged, though almost all patients did experience tarlatamab-related adverse events (94.8% for doses > 10 mg and 100% of patients with 10 mg doses). Overall, 66.4% of the total cohort experienced cytokine release syndrome of any grade, and 11.8% developed immune effector cell-associated neurotoxicity syndrome.
Discontinuation due to treatment-related adverse events occurred in 9 patients overall, and adverse events that led to dose interruption or reduction occurred in 32 patients overall.
“After many efforts at DLL3 targeting, we finally have an agent that shows activity and efficacy, and with convincing data,” said Jessica Menis, MD, a medical oncologist at the oncology department of the University Hospital of Verona, Italy, who was not involved in the study. The intracranial activity of tarlatamab “needs to be further evaluated in untreated patients,” Dr. Menis noted, because the study included only patients with stable, treated brain metastases.
And given the high rates of adverse events, Dr. Menis cautioned that adverse event management “will be a challenge.”
On X (Twitter), Tom Newsom-Davis, MBBS, PhD, a consultant in medical oncology at Chelsea and Westminster Hospital, London, said that tarlatamab is “not a straightforward drug to use,” highlighting the occurrence of cytokine release syndrome.
“But in this significantly pretreated population and in this hard-to-treat tumor type,” the rate and duration of responses seen with the extended follow-up are ‘impressive’,” he added.
DeLLphi-300, 301, and 304 were funded by Amgen Inc. Dr. Hummel declared relationships with several companies, including Amgen, Bristol Myers Squibb, AstraZeneca, Celgene, Merck, Novartis, Daiichi Sankyo, and Roche. Dr. Menis declared relationships with AstraZeneca, BMS, MSD, Roche, and Novartis.
A version of this article appeared on Medscape.com.
The investigational bispecific T-cell engager tarlatamab achieved durable responses and clinically meaningful survival outcomes in patients with small-cell lung cancer (SCLC), particularly at lower doses, according to a follow-up analysis of the phase 1 DeLLphi-300 trial.
Most patients with central nervous system tumors also sustained tumor shrinkage long after receiving radiotherapy, providing “encouraging evidence” of the new agent’s intracranial activity, said study presenter Horst-Dieter Hummel, MD, Comprehensive Cancer Center Mainfranken, Würzburg, Germany.
The research was presented at the European Lung Cancer Congress 2024 on March 22.
Data from the phase 1 and phase 2 DeLLphi trials, published last year, showed the compound achieved “encouraging clinical activity” in pretreated patients, said Dr. Hummel.
The initial phase 1 DeLLphi study found that after a median follow-up of 8.7 months, the immunotherapy led to a disease control rate of 51.4%, a median progression-free survival of 3.7 months, and median overall survival of 13.2 months.
At the meeting, Dr. Hummel reported longer-term outcomes from the phase 1 study over a median of 12.1 months as well as intracranial activity in patients who received clinically relevant doses of tarlatamab, defined as ≥ 10 mg.
The 152 patients included in the analysis had a median of two prior lines of therapy; 76.3% had undergone radiotherapy, and 63.2% had received immunotherapy. Liver metastases were present in 42.1% of patients, and 25.0% had brain metastases.
Doses varied among participants, with 76 patients (50.0%) receiving 100 mg, 32 (21.0%) receiving 100 mg via extended intravenous infusion, 17 (11.2%) receiving 10 mg, and 8 (5.3%) receiving 30 mg.
The overall objective response rate was 25.0%, with a median duration of response of 11.2 months. Among patients given the 10-mg dose, the objective response rate was higher, at 35.3%, as was the median duration of response, at 14.9 months.
Tarlatamab was associated with a median overall survival of 17.5 months, with 57.9% of patients alive at 12 months. Patients receiving the 10 mg dose had a better median overall survival of 20.3 months.
Of the 16 patients with analyzable central nervous system tumors, 62.5% experienced tumor shrinkage by ≥ 30% and 87.5% experienced intracranial disease control, which lasted for a median of 7.4 months.
In this follow-up study, tarlatamab demonstrated “clinically meaningful survival outcomes in patients with previously treated SCLC, particularly with the 10 mg dose,” Dr. Hummel concluded in his presentation.
No new safety signals emerged, though almost all patients did experience tarlatamab-related adverse events (94.8% for doses > 10 mg and 100% of patients with 10 mg doses). Overall, 66.4% of the total cohort experienced cytokine release syndrome of any grade, and 11.8% developed immune effector cell-associated neurotoxicity syndrome.
Discontinuation due to treatment-related adverse events occurred in 9 patients overall, and adverse events that led to dose interruption or reduction occurred in 32 patients overall.
“After many efforts at DLL3 targeting, we finally have an agent that shows activity and efficacy, and with convincing data,” said Jessica Menis, MD, a medical oncologist at the oncology department of the University Hospital of Verona, Italy, who was not involved in the study. The intracranial activity of tarlatamab “needs to be further evaluated in untreated patients,” Dr. Menis noted, because the study included only patients with stable, treated brain metastases.
And given the high rates of adverse events, Dr. Menis cautioned that adverse event management “will be a challenge.”
On X (Twitter), Tom Newsom-Davis, MBBS, PhD, a consultant in medical oncology at Chelsea and Westminster Hospital, London, said that tarlatamab is “not a straightforward drug to use,” highlighting the occurrence of cytokine release syndrome.
“But in this significantly pretreated population and in this hard-to-treat tumor type,” the rate and duration of responses seen with the extended follow-up are ‘impressive’,” he added.
DeLLphi-300, 301, and 304 were funded by Amgen Inc. Dr. Hummel declared relationships with several companies, including Amgen, Bristol Myers Squibb, AstraZeneca, Celgene, Merck, Novartis, Daiichi Sankyo, and Roche. Dr. Menis declared relationships with AstraZeneca, BMS, MSD, Roche, and Novartis.
A version of this article appeared on Medscape.com.
FROM ELCC 2024
FDA OKs First-in-Class Agent for Pulmonary Arterial Hypertension
The US Food and Drug Administration (FDA) has approved sotatercept (Winrevair, Merck), for the treatment of adults with pulmonary arterial hypertension (PAH), World Health Organization (WHO) Group 1, to increase exercise capacity, improve WHO functional class, and reduce the risk for clinical worsening events.
“Sotatercept added to background therapy has the potential to become a new standard-of-care option for patients with pulmonary arterial hypertension,” added coinvestigator Aaron B. Waxman, MD, PhD, executive director of the Center for Pulmonary Heart Diseases at Brigham and Women’s Hospital, Boston.
The approval was based on results of the phase 3 STELLAR study, a global, double-blind, placebo-controlled, multicenter, parallel-group clinical trial in which, 323 patients with PAH (WHO Group 1, functional class II or III) were randomly assigned 1:1 to add sotatercept or placebo to stable background therapy.
The results showed that sotatercept, administered subcutaneously every 3 weeks for 24 weeks, improved average 6-minute walk distance from baseline by a significant and clinically meaningful 40.8 meters compared with placebo for the trial’s primary efficacy endpoint (P < .001).
Sotatercept also led to significant improvement in multiple secondary outcome measures, including:
- Reduction in the risk for death from any cause or PAH clinical worsening events by 84% vs background therapy alone (number of events: 9 vs 42; hazard ratio [HR], 0.16; P < .001)
- Improvement in FC from baseline at 24 weeks in 29% of patients compared with 14% of patients treated with placebo (P < .001)
- Improvement in pulmonary vascular resistance (PVR), with an average 235 dyn/sec/cm5 reduction in PVR from baseline (P < .001)
- Improvement from baseline in N-terminal pro–B-type natriuretic peptide (NT-proBNP) levels. The median treatment difference in NT-proBNP between sotatercept and placebo was -442 pg/mL (P < .001)
The results were reported last year at the joint scientific sessions of the American College of Cardiology and the World Heart Federation, with simultaneous publication in The New England Journal of Medicine.
Sotatercept injection may be administered by patients or caregivers with guidance, training, and follow-up from a healthcare provider. The recommended starting dose is 0.3 mg/kg. The recommended target dose is 0.7 mg/kg every 3 weeks.
Sotatercept may increase hemoglobin, may lead to erythrocytosis, and may decrease platelet count and lead to severe thrombocytopenia. Treatment should not be initiated if platelet count is < 50,000/mm3.
Hemoglobin and platelets should be monitored before each dose of sotatercept for the first five doses, or longer if values are unstable, and periodically thereafter to determine if dose adjustments are required.
Full prescribing information is available online.
Merck estimates that sotatercept will be available for dispensing by select specialty pharmacies in the United States by the end of April 2024.
A version of this article appeared on Medscape.com.
The US Food and Drug Administration (FDA) has approved sotatercept (Winrevair, Merck), for the treatment of adults with pulmonary arterial hypertension (PAH), World Health Organization (WHO) Group 1, to increase exercise capacity, improve WHO functional class, and reduce the risk for clinical worsening events.
“Sotatercept added to background therapy has the potential to become a new standard-of-care option for patients with pulmonary arterial hypertension,” added coinvestigator Aaron B. Waxman, MD, PhD, executive director of the Center for Pulmonary Heart Diseases at Brigham and Women’s Hospital, Boston.
The approval was based on results of the phase 3 STELLAR study, a global, double-blind, placebo-controlled, multicenter, parallel-group clinical trial in which, 323 patients with PAH (WHO Group 1, functional class II or III) were randomly assigned 1:1 to add sotatercept or placebo to stable background therapy.
The results showed that sotatercept, administered subcutaneously every 3 weeks for 24 weeks, improved average 6-minute walk distance from baseline by a significant and clinically meaningful 40.8 meters compared with placebo for the trial’s primary efficacy endpoint (P < .001).
Sotatercept also led to significant improvement in multiple secondary outcome measures, including:
- Reduction in the risk for death from any cause or PAH clinical worsening events by 84% vs background therapy alone (number of events: 9 vs 42; hazard ratio [HR], 0.16; P < .001)
- Improvement in FC from baseline at 24 weeks in 29% of patients compared with 14% of patients treated with placebo (P < .001)
- Improvement in pulmonary vascular resistance (PVR), with an average 235 dyn/sec/cm5 reduction in PVR from baseline (P < .001)
- Improvement from baseline in N-terminal pro–B-type natriuretic peptide (NT-proBNP) levels. The median treatment difference in NT-proBNP between sotatercept and placebo was -442 pg/mL (P < .001)
The results were reported last year at the joint scientific sessions of the American College of Cardiology and the World Heart Federation, with simultaneous publication in The New England Journal of Medicine.
Sotatercept injection may be administered by patients or caregivers with guidance, training, and follow-up from a healthcare provider. The recommended starting dose is 0.3 mg/kg. The recommended target dose is 0.7 mg/kg every 3 weeks.
Sotatercept may increase hemoglobin, may lead to erythrocytosis, and may decrease platelet count and lead to severe thrombocytopenia. Treatment should not be initiated if platelet count is < 50,000/mm3.
Hemoglobin and platelets should be monitored before each dose of sotatercept for the first five doses, or longer if values are unstable, and periodically thereafter to determine if dose adjustments are required.
Full prescribing information is available online.
Merck estimates that sotatercept will be available for dispensing by select specialty pharmacies in the United States by the end of April 2024.
A version of this article appeared on Medscape.com.
The US Food and Drug Administration (FDA) has approved sotatercept (Winrevair, Merck), for the treatment of adults with pulmonary arterial hypertension (PAH), World Health Organization (WHO) Group 1, to increase exercise capacity, improve WHO functional class, and reduce the risk for clinical worsening events.
“Sotatercept added to background therapy has the potential to become a new standard-of-care option for patients with pulmonary arterial hypertension,” added coinvestigator Aaron B. Waxman, MD, PhD, executive director of the Center for Pulmonary Heart Diseases at Brigham and Women’s Hospital, Boston.
The approval was based on results of the phase 3 STELLAR study, a global, double-blind, placebo-controlled, multicenter, parallel-group clinical trial in which, 323 patients with PAH (WHO Group 1, functional class II or III) were randomly assigned 1:1 to add sotatercept or placebo to stable background therapy.
The results showed that sotatercept, administered subcutaneously every 3 weeks for 24 weeks, improved average 6-minute walk distance from baseline by a significant and clinically meaningful 40.8 meters compared with placebo for the trial’s primary efficacy endpoint (P < .001).
Sotatercept also led to significant improvement in multiple secondary outcome measures, including:
- Reduction in the risk for death from any cause or PAH clinical worsening events by 84% vs background therapy alone (number of events: 9 vs 42; hazard ratio [HR], 0.16; P < .001)
- Improvement in FC from baseline at 24 weeks in 29% of patients compared with 14% of patients treated with placebo (P < .001)
- Improvement in pulmonary vascular resistance (PVR), with an average 235 dyn/sec/cm5 reduction in PVR from baseline (P < .001)
- Improvement from baseline in N-terminal pro–B-type natriuretic peptide (NT-proBNP) levels. The median treatment difference in NT-proBNP between sotatercept and placebo was -442 pg/mL (P < .001)
The results were reported last year at the joint scientific sessions of the American College of Cardiology and the World Heart Federation, with simultaneous publication in The New England Journal of Medicine.
Sotatercept injection may be administered by patients or caregivers with guidance, training, and follow-up from a healthcare provider. The recommended starting dose is 0.3 mg/kg. The recommended target dose is 0.7 mg/kg every 3 weeks.
Sotatercept may increase hemoglobin, may lead to erythrocytosis, and may decrease platelet count and lead to severe thrombocytopenia. Treatment should not be initiated if platelet count is < 50,000/mm3.
Hemoglobin and platelets should be monitored before each dose of sotatercept for the first five doses, or longer if values are unstable, and periodically thereafter to determine if dose adjustments are required.
Full prescribing information is available online.
Merck estimates that sotatercept will be available for dispensing by select specialty pharmacies in the United States by the end of April 2024.
A version of this article appeared on Medscape.com.
Can Sweeteners Improve Weight Maintenance, Overeating?
TOPLINE:
, with no increases in type 2 diabetes or cardiovascular disease risk compared with a diet excluding the sweeteners, a randomized trial showed.
The study also showed that among overweight or obese children, greater reductions in uncontrolled eating were observed among those receiving the sweeteners.
The findings counter previous reports that raised concerns about the non-sugar sweeteners, including recent research from the World Health Organization suggesting no benefits in weight control and a possible increase in the risk for type 2 diabetes or cardiovascular disease with the sweeteners.
METHODOLOGY:
- The findings are from an exploratory analysis of the multicenter, randomized SWEET trial.
- The trial involved 341 adults with overweight or obesity (aged 18-65 years, 71% women, body mass index [BMI] ≥ 25) and 38 children with overweight (aged 6-12 years, 60% girls, BMI-for-age > 85th percentile), recruited in Denmark, Spain, Greece, and the Netherlands through webpages, social media, newspapers, and registries.
- For the first 2 months of the trial, adults were instructed to follow a low-energy diet (the Cambridge Weight Plan) with the goal of achieving at least 5% weight loss, while children received dietary advice to maintain body weight.
- In the subsequent 10 months, adults as well as children were randomized to healthy diets that either consisted of less than 10% of calories from added sugar but permitted foods and drinks with sweeteners and sweetness enhancers, or the same diet but not allowing the use of the sweeteners or sweetness enhancers.
- Participants had weight, BMI, anthropometry, and risk markers for type 2 diabetes and cardiovascular disease monitored at the trial’s baseline, as well as at 2, 6, and 12 months.
- In addition, participants completed food frequency questionnaires and provided urine samples to assess biomarkers of the sweeteners, fructose and sucrose, in order to measure compliance with the dietary instructions.
TAKEAWAY:
- While the sweetener and non-sweetener groups both had decreases in consumption of products high in sugar, the reduction was significantly higher in the group that allowed use of the sweeteners (P = .002).
- In the intention-to-treat analyses, adults (n = 277) permitted sweeteners showed a small but significantly greater weight loss maintenance after 1 year than the non-sweetener group (average weight loss, 7.2 kg vs 5.6 kg; P = .029).
- Among 203 participants who completed the trial, there were no differences between the groups in terms of markers for type 2 diabetes and cardiovascular disease.
- There were also no differences between the groups in terms of subjective appetite sensations and appetite hormones in a subgroup of 104 patients.
- In an analysis of 22 children who completed the study, there were no differences in BMI-for-age z scores between sweetener and non-sweetener groups.
- In terms of effects on eating behavior, adults in the sweetener group reported greater diet satisfaction when eating out (P = .03), increased positive mood (P = .013), and reduced craving for sweet food (P = .034) at 6 months than in the non-sweetener group.
- Conversely, those receiving no sweeteners had a greater liking bias for sweet vs savory foods at 6 months (P = .023) and 12 months (P = .005).
- There were no differences between the groups in reported physical activity or quality of life.
- However, among children with higher uncontrolled eating scores at baseline, the uncontrolled eating scores at 12 months were significantly lower among children who were allowed the sugar-substitute sweeteners vs the non-sweetener children (P = .021).
IN PRACTICE:
“Our findings suggest that the inclusion of low/no energy-sweetened products may benefit children who show high levels of uncontrolled eating,” said the study’s co-lead author, Clarissa Dakin, of the Appetite Control and Energy Balance Research Group at the University of Leeds, Leeds, England, in a press statement.
“Together, these findings provide important insights for the ongoing reevaluation of food additive sweeteners by the European Food Safety Authority and other health agencies worldwide,” she said.
Coauthor Jason Halford, head of the School of Psychology at the University of Leeds, added in the press statement that “the use of low-calorie sweeteners in weight management has been questioned, in part because of the link between their use and apparent weight gain in observational studies.”
“However, increasingly, it is becoming apparent that is not the case in long-term studies,” said a study co-author in a press statement.”
SOURCE:
The findings from the two abstracts will be presented in May at the European Association for the Study of Obesity. The study abstracts were issued in advance.
LIMITATIONS:
Some of the results, particularly in children’s subgroups, were limited by the relatively low number of children, underscoring the need for future studies on the issue, the authors noted.
DISCLOSURES:
Dr. Halford has received research funding from the American Beverage Association.
A version of this article appeared on Medscape.com.
TOPLINE:
, with no increases in type 2 diabetes or cardiovascular disease risk compared with a diet excluding the sweeteners, a randomized trial showed.
The study also showed that among overweight or obese children, greater reductions in uncontrolled eating were observed among those receiving the sweeteners.
The findings counter previous reports that raised concerns about the non-sugar sweeteners, including recent research from the World Health Organization suggesting no benefits in weight control and a possible increase in the risk for type 2 diabetes or cardiovascular disease with the sweeteners.
METHODOLOGY:
- The findings are from an exploratory analysis of the multicenter, randomized SWEET trial.
- The trial involved 341 adults with overweight or obesity (aged 18-65 years, 71% women, body mass index [BMI] ≥ 25) and 38 children with overweight (aged 6-12 years, 60% girls, BMI-for-age > 85th percentile), recruited in Denmark, Spain, Greece, and the Netherlands through webpages, social media, newspapers, and registries.
- For the first 2 months of the trial, adults were instructed to follow a low-energy diet (the Cambridge Weight Plan) with the goal of achieving at least 5% weight loss, while children received dietary advice to maintain body weight.
- In the subsequent 10 months, adults as well as children were randomized to healthy diets that either consisted of less than 10% of calories from added sugar but permitted foods and drinks with sweeteners and sweetness enhancers, or the same diet but not allowing the use of the sweeteners or sweetness enhancers.
- Participants had weight, BMI, anthropometry, and risk markers for type 2 diabetes and cardiovascular disease monitored at the trial’s baseline, as well as at 2, 6, and 12 months.
- In addition, participants completed food frequency questionnaires and provided urine samples to assess biomarkers of the sweeteners, fructose and sucrose, in order to measure compliance with the dietary instructions.
TAKEAWAY:
- While the sweetener and non-sweetener groups both had decreases in consumption of products high in sugar, the reduction was significantly higher in the group that allowed use of the sweeteners (P = .002).
- In the intention-to-treat analyses, adults (n = 277) permitted sweeteners showed a small but significantly greater weight loss maintenance after 1 year than the non-sweetener group (average weight loss, 7.2 kg vs 5.6 kg; P = .029).
- Among 203 participants who completed the trial, there were no differences between the groups in terms of markers for type 2 diabetes and cardiovascular disease.
- There were also no differences between the groups in terms of subjective appetite sensations and appetite hormones in a subgroup of 104 patients.
- In an analysis of 22 children who completed the study, there were no differences in BMI-for-age z scores between sweetener and non-sweetener groups.
- In terms of effects on eating behavior, adults in the sweetener group reported greater diet satisfaction when eating out (P = .03), increased positive mood (P = .013), and reduced craving for sweet food (P = .034) at 6 months than in the non-sweetener group.
- Conversely, those receiving no sweeteners had a greater liking bias for sweet vs savory foods at 6 months (P = .023) and 12 months (P = .005).
- There were no differences between the groups in reported physical activity or quality of life.
- However, among children with higher uncontrolled eating scores at baseline, the uncontrolled eating scores at 12 months were significantly lower among children who were allowed the sugar-substitute sweeteners vs the non-sweetener children (P = .021).
IN PRACTICE:
“Our findings suggest that the inclusion of low/no energy-sweetened products may benefit children who show high levels of uncontrolled eating,” said the study’s co-lead author, Clarissa Dakin, of the Appetite Control and Energy Balance Research Group at the University of Leeds, Leeds, England, in a press statement.
“Together, these findings provide important insights for the ongoing reevaluation of food additive sweeteners by the European Food Safety Authority and other health agencies worldwide,” she said.
Coauthor Jason Halford, head of the School of Psychology at the University of Leeds, added in the press statement that “the use of low-calorie sweeteners in weight management has been questioned, in part because of the link between their use and apparent weight gain in observational studies.”
“However, increasingly, it is becoming apparent that is not the case in long-term studies,” said a study co-author in a press statement.”
SOURCE:
The findings from the two abstracts will be presented in May at the European Association for the Study of Obesity. The study abstracts were issued in advance.
LIMITATIONS:
Some of the results, particularly in children’s subgroups, were limited by the relatively low number of children, underscoring the need for future studies on the issue, the authors noted.
DISCLOSURES:
Dr. Halford has received research funding from the American Beverage Association.
A version of this article appeared on Medscape.com.
TOPLINE:
, with no increases in type 2 diabetes or cardiovascular disease risk compared with a diet excluding the sweeteners, a randomized trial showed.
The study also showed that among overweight or obese children, greater reductions in uncontrolled eating were observed among those receiving the sweeteners.
The findings counter previous reports that raised concerns about the non-sugar sweeteners, including recent research from the World Health Organization suggesting no benefits in weight control and a possible increase in the risk for type 2 diabetes or cardiovascular disease with the sweeteners.
METHODOLOGY:
- The findings are from an exploratory analysis of the multicenter, randomized SWEET trial.
- The trial involved 341 adults with overweight or obesity (aged 18-65 years, 71% women, body mass index [BMI] ≥ 25) and 38 children with overweight (aged 6-12 years, 60% girls, BMI-for-age > 85th percentile), recruited in Denmark, Spain, Greece, and the Netherlands through webpages, social media, newspapers, and registries.
- For the first 2 months of the trial, adults were instructed to follow a low-energy diet (the Cambridge Weight Plan) with the goal of achieving at least 5% weight loss, while children received dietary advice to maintain body weight.
- In the subsequent 10 months, adults as well as children were randomized to healthy diets that either consisted of less than 10% of calories from added sugar but permitted foods and drinks with sweeteners and sweetness enhancers, or the same diet but not allowing the use of the sweeteners or sweetness enhancers.
- Participants had weight, BMI, anthropometry, and risk markers for type 2 diabetes and cardiovascular disease monitored at the trial’s baseline, as well as at 2, 6, and 12 months.
- In addition, participants completed food frequency questionnaires and provided urine samples to assess biomarkers of the sweeteners, fructose and sucrose, in order to measure compliance with the dietary instructions.
TAKEAWAY:
- While the sweetener and non-sweetener groups both had decreases in consumption of products high in sugar, the reduction was significantly higher in the group that allowed use of the sweeteners (P = .002).
- In the intention-to-treat analyses, adults (n = 277) permitted sweeteners showed a small but significantly greater weight loss maintenance after 1 year than the non-sweetener group (average weight loss, 7.2 kg vs 5.6 kg; P = .029).
- Among 203 participants who completed the trial, there were no differences between the groups in terms of markers for type 2 diabetes and cardiovascular disease.
- There were also no differences between the groups in terms of subjective appetite sensations and appetite hormones in a subgroup of 104 patients.
- In an analysis of 22 children who completed the study, there were no differences in BMI-for-age z scores between sweetener and non-sweetener groups.
- In terms of effects on eating behavior, adults in the sweetener group reported greater diet satisfaction when eating out (P = .03), increased positive mood (P = .013), and reduced craving for sweet food (P = .034) at 6 months than in the non-sweetener group.
- Conversely, those receiving no sweeteners had a greater liking bias for sweet vs savory foods at 6 months (P = .023) and 12 months (P = .005).
- There were no differences between the groups in reported physical activity or quality of life.
- However, among children with higher uncontrolled eating scores at baseline, the uncontrolled eating scores at 12 months were significantly lower among children who were allowed the sugar-substitute sweeteners vs the non-sweetener children (P = .021).
IN PRACTICE:
“Our findings suggest that the inclusion of low/no energy-sweetened products may benefit children who show high levels of uncontrolled eating,” said the study’s co-lead author, Clarissa Dakin, of the Appetite Control and Energy Balance Research Group at the University of Leeds, Leeds, England, in a press statement.
“Together, these findings provide important insights for the ongoing reevaluation of food additive sweeteners by the European Food Safety Authority and other health agencies worldwide,” she said.
Coauthor Jason Halford, head of the School of Psychology at the University of Leeds, added in the press statement that “the use of low-calorie sweeteners in weight management has been questioned, in part because of the link between their use and apparent weight gain in observational studies.”
“However, increasingly, it is becoming apparent that is not the case in long-term studies,” said a study co-author in a press statement.”
SOURCE:
The findings from the two abstracts will be presented in May at the European Association for the Study of Obesity. The study abstracts were issued in advance.
LIMITATIONS:
Some of the results, particularly in children’s subgroups, were limited by the relatively low number of children, underscoring the need for future studies on the issue, the authors noted.
DISCLOSURES:
Dr. Halford has received research funding from the American Beverage Association.
A version of this article appeared on Medscape.com.
This Could Be Key to Motivating Older Patients to Exercise
Starting an exercise regimen with others can be a powerful fitness motivator, and new research spotlights the strategy’s particular importance for older adults.
In a randomized clinical trial published in JAMA Network Open, older adults who talked with peers about their exercise program were able to increase and sustain physical activity levels much better than those who focused on self-motivation and setting fitness goals.
Such self-focused — or “intrapersonal” — strategies tend to be more common in health and fitness than interactive, or “interpersonal,” ones, the study authors noted. Yet, research on their effectiveness is limited.
“We’re not saying that intrapersonal strategies should not be used,” said study author Siobhan McMahon, PhD, associate professor and codirector of the Center on Aging Science and Care at the University of Minnesota, in Minneapolis, Minnesota, “but this study shows that interpersonal strategies are really important.”
Low physical activity among older adults is linked with “disability, difficulty managing chronic conditions, and increased falls and related injuries,” the authors wrote. Exercise can be the antidote, yet fewer than 16% of older adults meet the recommended guidelines (150 minutes of moderate aerobic activity and two muscle-strengthening sessions per week).
The study builds on previous research that suggests interpersonal strategies could help change that by encouraging more older adults to move.
Intrapersonal vs Interpersonal Behavior Change Strategies
More than 300 participants aged 70 years and older who did not meet physical activity guidelines were given a wearable fitness tracker and an exercise program and randomly split into four groups:
- One using intrapersonal behavior change strategies
- Another using interpersonal strategies
- A group combining both intrapersonal and interpersonal strategies
- A control group that received neither intervention
For 8 weeks, all participants exercised in meet-ups and discussed their progress in their groups. Afterward, they were left to their own devices and monitored for the remainder of the year.
“The intrapersonal strategies group involved personal reflection,” said Dr. McMahon. They set personal goals (increasing daily step count or exercise repetitions) and developed action plans for implementing physical activity into their daily routines.
“The interpersonal group involved more peer-to-peer conversation, collaborative learning, and sharing,” said Dr. McMahon. Participants talked among themselves about how they could sustain doing the prescribed exercises at home. “Through those conversations, they learned and experimented,” Dr. McMahon said. They problem-solved, determining what barriers might stop them from exercising and brainstorming ways around them.
The researchers evaluated the participants after 1 week, 6 months, and 12 months. The interpersonal group exhibited significant increases in physical activity — including light, moderate, and vigorous activity — for the entire year. They increased their average physical activity per day by 21-28 minutes and their daily step count by 776-1058.
The intrapersonal group, meanwhile, exhibited no significant changes in total physical activity. (The third experimental group, the intrapersonal plus interpersonal condition, had results similar to the interpersonal one.)
The results echoed the findings of a similar study Dr. McMahon conducted in 2017. “We followed people over a longer period of time in this [new] study,” she said, “12 months instead of 6 months. This is important in physical activity studies because a lot of evidence shows that after 6 months, people’s activity drops off.”
How Socializing Promotes Exercise Compliance
Research on the effectiveness of exercise in social groups dates back as far as the 19th century. It’s called the social facilitation theory: The idea that people will make an increased effort as a result of the real, imagined, or implied presence of others.
“Norman Triplett was a scientist who studied indoor cyclists, and he came up with the social facilitation theory in 1898,” said Robert Linkul, CSCS*D, who sits on the National Strength and Conditioning Association’s board of directors and specializes in exercise for older adults. “He noticed that during relays, the first cyclist would get slower as he fatigued, but as soon as his teammate came out, his last lap would be faster than his previous two laps. People try harder when there’s some other person present. They tend to feel pressure to perform because they don’t want to look bad.”
Dr. McMahon said the exact psychology of why socializing supports exercise isn’t clear yet but noted that talking to other people builds relationships and makes one feel connected to and involved with a community.
“I think connections between peers are really important,” said Dr. McMahon. “It goes beyond just being in the same room and doing the exercises together. It’s taking a little bit of time to talk about it. To acknowledge what they’re doing and their progress. To encourage each other and provide support.”
Some of the study participants even became friends and continued to meet on their own time over the course of the trial.
“They stayed in touch,” said Dr. McMahon. “One thing that people talked about after the study, even if they weren’t friends, was that the conversations within the meetings made them feel kind of a fellowship that helped them learn about themselves or people like them.”
Help Patients Find Their Own Fellowship of Active People
- Communicate the importance of exercise. During appointments, ask how the patient is doing with their exercise and listen for any obstacles to compliance, Dr. McMahon said.
- See if they have access to fitness classes. Many community-dwelling older adults do, Mr. Linkul said. If not, consider local or state agencies on aging — “in Minnesota, we have a program, Juniper,” Dr. McMahon said, that maintains a list of physical activity programs — or AARP’s free online group classes, or Silver Sneakers (free for those with eligible Medicare Advantage plans).
- Reach out to local qualified fitness professionals. Trainers with the Training the Older Adult certification (founded by Mr. Linkul) can be found here. Other qualified trainers can be found through the Functional Aging Institute, American Council on Exercise, and National Academy of Sports Medicine, Mr. Linkul said. “Many of these trainers will offer semiprivate sessions,” said Mr. Linkul, “which is usually four to eight people.” Groups of this size often facilitate better participation than larger classes. “You get more personalized attention from the instructor along with an environment that allows social engagement,” said Mr. Linkul. If you have exercise or rehab professionals in your network, you might consider reaching out to them. Some physical therapists lead activity groups, though reimbursement challenges mean they aren’t common, Dr. McMahon said.
- Prescribe short walks with a friend, family member, or neighbor. Have the person start with 30 minutes of walking or rucking (walking with a weighted backpack) most days, Mr. Linkul suggested, a recommendation that is echoed by the American College of Sports Medicine.
- Encourage patients to talk about their exercise. Even for those who prefer to exercise solo, “our studies suggest it might be helpful to have conversations with others about movement, and motivations for movement,” Dr. McMahon said. They can simply mention one idea, question, or observation related to physical activity during casual catchups or chats.
- Recommend resistance training. That goes for patients with preexisting health conditions too, Mr. Linkul said. Physicians “find out a patient has low bone mineral density, and they’ll often tell them not to pick up anything heavy because they’ll hurt themselves — and that’s the exact wrong answer,” Mr. Linkul said. A total of 32% of the participants in the JAMA Network study had cardiovascular disease, nearly 34% had osteoporosis, 70% had arthritis, and more than 20% were living with diabetes.
- Expect pushback. Encouraging older adults to exercise is hard because many are resistant to it, Mr. Linkul acknowledged. Do it anyway. Some will listen and that makes the effort worthwhile. “I try to provide as much information as I can about what happens to aging bodies if they don’t train,” said Mr. Linkul. “These people are more likely to fall, they’ll die earlier, and have a poorer quality of life. But when they start exercising, they feel better immediately.”
A version of this article appeared on Medscape.com.
Starting an exercise regimen with others can be a powerful fitness motivator, and new research spotlights the strategy’s particular importance for older adults.
In a randomized clinical trial published in JAMA Network Open, older adults who talked with peers about their exercise program were able to increase and sustain physical activity levels much better than those who focused on self-motivation and setting fitness goals.
Such self-focused — or “intrapersonal” — strategies tend to be more common in health and fitness than interactive, or “interpersonal,” ones, the study authors noted. Yet, research on their effectiveness is limited.
“We’re not saying that intrapersonal strategies should not be used,” said study author Siobhan McMahon, PhD, associate professor and codirector of the Center on Aging Science and Care at the University of Minnesota, in Minneapolis, Minnesota, “but this study shows that interpersonal strategies are really important.”
Low physical activity among older adults is linked with “disability, difficulty managing chronic conditions, and increased falls and related injuries,” the authors wrote. Exercise can be the antidote, yet fewer than 16% of older adults meet the recommended guidelines (150 minutes of moderate aerobic activity and two muscle-strengthening sessions per week).
The study builds on previous research that suggests interpersonal strategies could help change that by encouraging more older adults to move.
Intrapersonal vs Interpersonal Behavior Change Strategies
More than 300 participants aged 70 years and older who did not meet physical activity guidelines were given a wearable fitness tracker and an exercise program and randomly split into four groups:
- One using intrapersonal behavior change strategies
- Another using interpersonal strategies
- A group combining both intrapersonal and interpersonal strategies
- A control group that received neither intervention
For 8 weeks, all participants exercised in meet-ups and discussed their progress in their groups. Afterward, they were left to their own devices and monitored for the remainder of the year.
“The intrapersonal strategies group involved personal reflection,” said Dr. McMahon. They set personal goals (increasing daily step count or exercise repetitions) and developed action plans for implementing physical activity into their daily routines.
“The interpersonal group involved more peer-to-peer conversation, collaborative learning, and sharing,” said Dr. McMahon. Participants talked among themselves about how they could sustain doing the prescribed exercises at home. “Through those conversations, they learned and experimented,” Dr. McMahon said. They problem-solved, determining what barriers might stop them from exercising and brainstorming ways around them.
The researchers evaluated the participants after 1 week, 6 months, and 12 months. The interpersonal group exhibited significant increases in physical activity — including light, moderate, and vigorous activity — for the entire year. They increased their average physical activity per day by 21-28 minutes and their daily step count by 776-1058.
The intrapersonal group, meanwhile, exhibited no significant changes in total physical activity. (The third experimental group, the intrapersonal plus interpersonal condition, had results similar to the interpersonal one.)
The results echoed the findings of a similar study Dr. McMahon conducted in 2017. “We followed people over a longer period of time in this [new] study,” she said, “12 months instead of 6 months. This is important in physical activity studies because a lot of evidence shows that after 6 months, people’s activity drops off.”
How Socializing Promotes Exercise Compliance
Research on the effectiveness of exercise in social groups dates back as far as the 19th century. It’s called the social facilitation theory: The idea that people will make an increased effort as a result of the real, imagined, or implied presence of others.
“Norman Triplett was a scientist who studied indoor cyclists, and he came up with the social facilitation theory in 1898,” said Robert Linkul, CSCS*D, who sits on the National Strength and Conditioning Association’s board of directors and specializes in exercise for older adults. “He noticed that during relays, the first cyclist would get slower as he fatigued, but as soon as his teammate came out, his last lap would be faster than his previous two laps. People try harder when there’s some other person present. They tend to feel pressure to perform because they don’t want to look bad.”
Dr. McMahon said the exact psychology of why socializing supports exercise isn’t clear yet but noted that talking to other people builds relationships and makes one feel connected to and involved with a community.
“I think connections between peers are really important,” said Dr. McMahon. “It goes beyond just being in the same room and doing the exercises together. It’s taking a little bit of time to talk about it. To acknowledge what they’re doing and their progress. To encourage each other and provide support.”
Some of the study participants even became friends and continued to meet on their own time over the course of the trial.
“They stayed in touch,” said Dr. McMahon. “One thing that people talked about after the study, even if they weren’t friends, was that the conversations within the meetings made them feel kind of a fellowship that helped them learn about themselves or people like them.”
Help Patients Find Their Own Fellowship of Active People
- Communicate the importance of exercise. During appointments, ask how the patient is doing with their exercise and listen for any obstacles to compliance, Dr. McMahon said.
- See if they have access to fitness classes. Many community-dwelling older adults do, Mr. Linkul said. If not, consider local or state agencies on aging — “in Minnesota, we have a program, Juniper,” Dr. McMahon said, that maintains a list of physical activity programs — or AARP’s free online group classes, or Silver Sneakers (free for those with eligible Medicare Advantage plans).
- Reach out to local qualified fitness professionals. Trainers with the Training the Older Adult certification (founded by Mr. Linkul) can be found here. Other qualified trainers can be found through the Functional Aging Institute, American Council on Exercise, and National Academy of Sports Medicine, Mr. Linkul said. “Many of these trainers will offer semiprivate sessions,” said Mr. Linkul, “which is usually four to eight people.” Groups of this size often facilitate better participation than larger classes. “You get more personalized attention from the instructor along with an environment that allows social engagement,” said Mr. Linkul. If you have exercise or rehab professionals in your network, you might consider reaching out to them. Some physical therapists lead activity groups, though reimbursement challenges mean they aren’t common, Dr. McMahon said.
- Prescribe short walks with a friend, family member, or neighbor. Have the person start with 30 minutes of walking or rucking (walking with a weighted backpack) most days, Mr. Linkul suggested, a recommendation that is echoed by the American College of Sports Medicine.
- Encourage patients to talk about their exercise. Even for those who prefer to exercise solo, “our studies suggest it might be helpful to have conversations with others about movement, and motivations for movement,” Dr. McMahon said. They can simply mention one idea, question, or observation related to physical activity during casual catchups or chats.
- Recommend resistance training. That goes for patients with preexisting health conditions too, Mr. Linkul said. Physicians “find out a patient has low bone mineral density, and they’ll often tell them not to pick up anything heavy because they’ll hurt themselves — and that’s the exact wrong answer,” Mr. Linkul said. A total of 32% of the participants in the JAMA Network study had cardiovascular disease, nearly 34% had osteoporosis, 70% had arthritis, and more than 20% were living with diabetes.
- Expect pushback. Encouraging older adults to exercise is hard because many are resistant to it, Mr. Linkul acknowledged. Do it anyway. Some will listen and that makes the effort worthwhile. “I try to provide as much information as I can about what happens to aging bodies if they don’t train,” said Mr. Linkul. “These people are more likely to fall, they’ll die earlier, and have a poorer quality of life. But when they start exercising, they feel better immediately.”
A version of this article appeared on Medscape.com.
Starting an exercise regimen with others can be a powerful fitness motivator, and new research spotlights the strategy’s particular importance for older adults.
In a randomized clinical trial published in JAMA Network Open, older adults who talked with peers about their exercise program were able to increase and sustain physical activity levels much better than those who focused on self-motivation and setting fitness goals.
Such self-focused — or “intrapersonal” — strategies tend to be more common in health and fitness than interactive, or “interpersonal,” ones, the study authors noted. Yet, research on their effectiveness is limited.
“We’re not saying that intrapersonal strategies should not be used,” said study author Siobhan McMahon, PhD, associate professor and codirector of the Center on Aging Science and Care at the University of Minnesota, in Minneapolis, Minnesota, “but this study shows that interpersonal strategies are really important.”
Low physical activity among older adults is linked with “disability, difficulty managing chronic conditions, and increased falls and related injuries,” the authors wrote. Exercise can be the antidote, yet fewer than 16% of older adults meet the recommended guidelines (150 minutes of moderate aerobic activity and two muscle-strengthening sessions per week).
The study builds on previous research that suggests interpersonal strategies could help change that by encouraging more older adults to move.
Intrapersonal vs Interpersonal Behavior Change Strategies
More than 300 participants aged 70 years and older who did not meet physical activity guidelines were given a wearable fitness tracker and an exercise program and randomly split into four groups:
- One using intrapersonal behavior change strategies
- Another using interpersonal strategies
- A group combining both intrapersonal and interpersonal strategies
- A control group that received neither intervention
For 8 weeks, all participants exercised in meet-ups and discussed their progress in their groups. Afterward, they were left to their own devices and monitored for the remainder of the year.
“The intrapersonal strategies group involved personal reflection,” said Dr. McMahon. They set personal goals (increasing daily step count or exercise repetitions) and developed action plans for implementing physical activity into their daily routines.
“The interpersonal group involved more peer-to-peer conversation, collaborative learning, and sharing,” said Dr. McMahon. Participants talked among themselves about how they could sustain doing the prescribed exercises at home. “Through those conversations, they learned and experimented,” Dr. McMahon said. They problem-solved, determining what barriers might stop them from exercising and brainstorming ways around them.
The researchers evaluated the participants after 1 week, 6 months, and 12 months. The interpersonal group exhibited significant increases in physical activity — including light, moderate, and vigorous activity — for the entire year. They increased their average physical activity per day by 21-28 minutes and their daily step count by 776-1058.
The intrapersonal group, meanwhile, exhibited no significant changes in total physical activity. (The third experimental group, the intrapersonal plus interpersonal condition, had results similar to the interpersonal one.)
The results echoed the findings of a similar study Dr. McMahon conducted in 2017. “We followed people over a longer period of time in this [new] study,” she said, “12 months instead of 6 months. This is important in physical activity studies because a lot of evidence shows that after 6 months, people’s activity drops off.”
How Socializing Promotes Exercise Compliance
Research on the effectiveness of exercise in social groups dates back as far as the 19th century. It’s called the social facilitation theory: The idea that people will make an increased effort as a result of the real, imagined, or implied presence of others.
“Norman Triplett was a scientist who studied indoor cyclists, and he came up with the social facilitation theory in 1898,” said Robert Linkul, CSCS*D, who sits on the National Strength and Conditioning Association’s board of directors and specializes in exercise for older adults. “He noticed that during relays, the first cyclist would get slower as he fatigued, but as soon as his teammate came out, his last lap would be faster than his previous two laps. People try harder when there’s some other person present. They tend to feel pressure to perform because they don’t want to look bad.”
Dr. McMahon said the exact psychology of why socializing supports exercise isn’t clear yet but noted that talking to other people builds relationships and makes one feel connected to and involved with a community.
“I think connections between peers are really important,” said Dr. McMahon. “It goes beyond just being in the same room and doing the exercises together. It’s taking a little bit of time to talk about it. To acknowledge what they’re doing and their progress. To encourage each other and provide support.”
Some of the study participants even became friends and continued to meet on their own time over the course of the trial.
“They stayed in touch,” said Dr. McMahon. “One thing that people talked about after the study, even if they weren’t friends, was that the conversations within the meetings made them feel kind of a fellowship that helped them learn about themselves or people like them.”
Help Patients Find Their Own Fellowship of Active People
- Communicate the importance of exercise. During appointments, ask how the patient is doing with their exercise and listen for any obstacles to compliance, Dr. McMahon said.
- See if they have access to fitness classes. Many community-dwelling older adults do, Mr. Linkul said. If not, consider local or state agencies on aging — “in Minnesota, we have a program, Juniper,” Dr. McMahon said, that maintains a list of physical activity programs — or AARP’s free online group classes, or Silver Sneakers (free for those with eligible Medicare Advantage plans).
- Reach out to local qualified fitness professionals. Trainers with the Training the Older Adult certification (founded by Mr. Linkul) can be found here. Other qualified trainers can be found through the Functional Aging Institute, American Council on Exercise, and National Academy of Sports Medicine, Mr. Linkul said. “Many of these trainers will offer semiprivate sessions,” said Mr. Linkul, “which is usually four to eight people.” Groups of this size often facilitate better participation than larger classes. “You get more personalized attention from the instructor along with an environment that allows social engagement,” said Mr. Linkul. If you have exercise or rehab professionals in your network, you might consider reaching out to them. Some physical therapists lead activity groups, though reimbursement challenges mean they aren’t common, Dr. McMahon said.
- Prescribe short walks with a friend, family member, or neighbor. Have the person start with 30 minutes of walking or rucking (walking with a weighted backpack) most days, Mr. Linkul suggested, a recommendation that is echoed by the American College of Sports Medicine.
- Encourage patients to talk about their exercise. Even for those who prefer to exercise solo, “our studies suggest it might be helpful to have conversations with others about movement, and motivations for movement,” Dr. McMahon said. They can simply mention one idea, question, or observation related to physical activity during casual catchups or chats.
- Recommend resistance training. That goes for patients with preexisting health conditions too, Mr. Linkul said. Physicians “find out a patient has low bone mineral density, and they’ll often tell them not to pick up anything heavy because they’ll hurt themselves — and that’s the exact wrong answer,” Mr. Linkul said. A total of 32% of the participants in the JAMA Network study had cardiovascular disease, nearly 34% had osteoporosis, 70% had arthritis, and more than 20% were living with diabetes.
- Expect pushback. Encouraging older adults to exercise is hard because many are resistant to it, Mr. Linkul acknowledged. Do it anyway. Some will listen and that makes the effort worthwhile. “I try to provide as much information as I can about what happens to aging bodies if they don’t train,” said Mr. Linkul. “These people are more likely to fall, they’ll die earlier, and have a poorer quality of life. But when they start exercising, they feel better immediately.”
A version of this article appeared on Medscape.com.
FROM JAMA NETWORK OPEN
Time Is Money: Should Physicians Be Compensated for EHR Engagement?
Electronic health records (EHRs) make providing coordinated, efficient care easier and reduce medical errors and test duplications; research has also correlated EHR adoption with higher patient satisfaction and outcomes. However, for physicians, the benefits come at a cost.
“I spend at least the same amount of time in the portal that I do in scheduled clinical time with patients,” said Eve Rittenberg, MD, primary care physician at Brigham and Women’s Hospital and assistant professor at Harvard Medical School, Boston, Massachusetts. “So, if I have a 4-hour session of seeing patients, I spend at least another 4 or more hours in the patient portal.”
The latest data showed that primary care physicians logged a median of 36.2 minutes in the healthcare portal per patient visit, spending 58.9% more time on orders, 24.4% more time reading and responding to messages, and 13% more time on chart review compared to prepandemic portal use.
“EHRs can be very powerful tools,” said Ralph DeBiasi, MD, a clinical cardiac electrophysiologist at Yale New Haven Health. “We’re still working on how to best harness that power to make us better doctors and better care teams and to take better care of our patients because their use can take up a lot of time.”
Portal Time Isn’t Paid Time
Sharp increases in the amount of time spent in the EHR responding to messages or dispensing medical advice via the portal often aren’t linked to increases in compensation; most portal time is unpaid.
“There isn’t specific time allocated to working in the portal; it’s either done in the office while a patient is sitting in an exam room or in the mornings and evenings outside of traditional working hours,” Dr. DeBiasi said. “I think it’s reasonable to consider it being reimbursed because we’re taking our time and effort and making decisions to help the patient.”
Compensation for portal time affects all physicians, but the degree of impact depends on their specialties. Primary care physicians spent significantly more daily and after-hours time in the EHR, entering notes and orders, and doing clinical reviews compared to surgical and medical specialties.
In addition to the outsized impact on primary care, physician compensation for portal time is also an equity issue.
Dr. Rittenberg researched the issue and found a higher volume of communication from both patients and staff to female physicians than male physicians. As a result, female physicians spend 41.4 minutes more on the EHR than their male counterparts, which equates to more unpaid time. It’s likely no coincidence then that burnout rates are also higher among female physicians, who also leave the clinical workforce in higher numbers, especially in primary care.
“Finding ways to fairly compensate physicians for their work also will address some of the equity issues in workload and the consequences,” Dr. Rittenberg said.
Addressing the Issue
Some health systems have started charging patients who seek medical advice via patient portals, equating the communication to asynchronous acute care or an additional care touchpoint and billing based on the length and complexity of the messages. Patient fees for seeking medical advice via portals vary widely depending on their health system and insurance.
At University of California San Francisco Health, billing patients for EHR communication led to a sharp decrease in patient messages, which eased physician workload. At Cleveland Clinic, physicians receive “productivity credits” for the time spent in the EHR that can be used to reduce their clinic hours (but have no impact on their compensation).
Changes to the Medicare Physician Fee Schedule also allow physicians to bill for “digital evaluation and management” based on the time spent in an EHR responding to patient-initiated questions and requests.
However, more efforts are needed to ease burnout and reverse the number of physicians who are seeing fewer patients or leaving medical practice altogether as a direct result of spending increasing amounts of unpaid time in the EHR. Dr. Rittenberg, who spends an estimated 50% of her working hours in the portal, had to reduce her clinical workload by 25% due to such heavy portal requirements.
“The workload has become unsustainable,” she said. “The work has undergone a dramatic change over the past decade, and the compensation system has not kept up with that change.”
Prioritizing Patient and Physician Experiences
The ever-expanding use of EHR is a result of their value as a healthcare tool. Data showed that the electronic exchange of information between patients and physicians improves diagnostics, reduces medical errors, enhances communication, and leads to more patient-centered care — and physicians want their patients to use the portal to maximize their healthcare.
“[The EHR] is good for patients,” said Dr. DeBiasi. “Sometimes, patients have access issues with healthcare, whether that’s not knowing what number to call or getting the right message to the right person at the right office. If [the portal] is good for them and helps them get access to care, we should embrace that and figure out a way to work it into our day-to-day schedules.”
But maximizing the patient experience shouldn’t come at the physicians’ expense. Dr. Rittenberg advocates a model that compensates physicians for the time spent in the EHR and prioritizes a team approach to rebalance the EHR workload to ensure that physicians aren’t devoting too much time to administrative tasks and can, instead, focus their time on clinical tasks.
“The way in which we provide healthcare has fundamentally shifted, and compensation models need to reflect that new reality,” Dr. Rittenberg added.
A version of this article appeared on Medscape.com.
Electronic health records (EHRs) make providing coordinated, efficient care easier and reduce medical errors and test duplications; research has also correlated EHR adoption with higher patient satisfaction and outcomes. However, for physicians, the benefits come at a cost.
“I spend at least the same amount of time in the portal that I do in scheduled clinical time with patients,” said Eve Rittenberg, MD, primary care physician at Brigham and Women’s Hospital and assistant professor at Harvard Medical School, Boston, Massachusetts. “So, if I have a 4-hour session of seeing patients, I spend at least another 4 or more hours in the patient portal.”
The latest data showed that primary care physicians logged a median of 36.2 minutes in the healthcare portal per patient visit, spending 58.9% more time on orders, 24.4% more time reading and responding to messages, and 13% more time on chart review compared to prepandemic portal use.
“EHRs can be very powerful tools,” said Ralph DeBiasi, MD, a clinical cardiac electrophysiologist at Yale New Haven Health. “We’re still working on how to best harness that power to make us better doctors and better care teams and to take better care of our patients because their use can take up a lot of time.”
Portal Time Isn’t Paid Time
Sharp increases in the amount of time spent in the EHR responding to messages or dispensing medical advice via the portal often aren’t linked to increases in compensation; most portal time is unpaid.
“There isn’t specific time allocated to working in the portal; it’s either done in the office while a patient is sitting in an exam room or in the mornings and evenings outside of traditional working hours,” Dr. DeBiasi said. “I think it’s reasonable to consider it being reimbursed because we’re taking our time and effort and making decisions to help the patient.”
Compensation for portal time affects all physicians, but the degree of impact depends on their specialties. Primary care physicians spent significantly more daily and after-hours time in the EHR, entering notes and orders, and doing clinical reviews compared to surgical and medical specialties.
In addition to the outsized impact on primary care, physician compensation for portal time is also an equity issue.
Dr. Rittenberg researched the issue and found a higher volume of communication from both patients and staff to female physicians than male physicians. As a result, female physicians spend 41.4 minutes more on the EHR than their male counterparts, which equates to more unpaid time. It’s likely no coincidence then that burnout rates are also higher among female physicians, who also leave the clinical workforce in higher numbers, especially in primary care.
“Finding ways to fairly compensate physicians for their work also will address some of the equity issues in workload and the consequences,” Dr. Rittenberg said.
Addressing the Issue
Some health systems have started charging patients who seek medical advice via patient portals, equating the communication to asynchronous acute care or an additional care touchpoint and billing based on the length and complexity of the messages. Patient fees for seeking medical advice via portals vary widely depending on their health system and insurance.
At University of California San Francisco Health, billing patients for EHR communication led to a sharp decrease in patient messages, which eased physician workload. At Cleveland Clinic, physicians receive “productivity credits” for the time spent in the EHR that can be used to reduce their clinic hours (but have no impact on their compensation).
Changes to the Medicare Physician Fee Schedule also allow physicians to bill for “digital evaluation and management” based on the time spent in an EHR responding to patient-initiated questions and requests.
However, more efforts are needed to ease burnout and reverse the number of physicians who are seeing fewer patients or leaving medical practice altogether as a direct result of spending increasing amounts of unpaid time in the EHR. Dr. Rittenberg, who spends an estimated 50% of her working hours in the portal, had to reduce her clinical workload by 25% due to such heavy portal requirements.
“The workload has become unsustainable,” she said. “The work has undergone a dramatic change over the past decade, and the compensation system has not kept up with that change.”
Prioritizing Patient and Physician Experiences
The ever-expanding use of EHR is a result of their value as a healthcare tool. Data showed that the electronic exchange of information between patients and physicians improves diagnostics, reduces medical errors, enhances communication, and leads to more patient-centered care — and physicians want their patients to use the portal to maximize their healthcare.
“[The EHR] is good for patients,” said Dr. DeBiasi. “Sometimes, patients have access issues with healthcare, whether that’s not knowing what number to call or getting the right message to the right person at the right office. If [the portal] is good for them and helps them get access to care, we should embrace that and figure out a way to work it into our day-to-day schedules.”
But maximizing the patient experience shouldn’t come at the physicians’ expense. Dr. Rittenberg advocates a model that compensates physicians for the time spent in the EHR and prioritizes a team approach to rebalance the EHR workload to ensure that physicians aren’t devoting too much time to administrative tasks and can, instead, focus their time on clinical tasks.
“The way in which we provide healthcare has fundamentally shifted, and compensation models need to reflect that new reality,” Dr. Rittenberg added.
A version of this article appeared on Medscape.com.
Electronic health records (EHRs) make providing coordinated, efficient care easier and reduce medical errors and test duplications; research has also correlated EHR adoption with higher patient satisfaction and outcomes. However, for physicians, the benefits come at a cost.
“I spend at least the same amount of time in the portal that I do in scheduled clinical time with patients,” said Eve Rittenberg, MD, primary care physician at Brigham and Women’s Hospital and assistant professor at Harvard Medical School, Boston, Massachusetts. “So, if I have a 4-hour session of seeing patients, I spend at least another 4 or more hours in the patient portal.”
The latest data showed that primary care physicians logged a median of 36.2 minutes in the healthcare portal per patient visit, spending 58.9% more time on orders, 24.4% more time reading and responding to messages, and 13% more time on chart review compared to prepandemic portal use.
“EHRs can be very powerful tools,” said Ralph DeBiasi, MD, a clinical cardiac electrophysiologist at Yale New Haven Health. “We’re still working on how to best harness that power to make us better doctors and better care teams and to take better care of our patients because their use can take up a lot of time.”
Portal Time Isn’t Paid Time
Sharp increases in the amount of time spent in the EHR responding to messages or dispensing medical advice via the portal often aren’t linked to increases in compensation; most portal time is unpaid.
“There isn’t specific time allocated to working in the portal; it’s either done in the office while a patient is sitting in an exam room or in the mornings and evenings outside of traditional working hours,” Dr. DeBiasi said. “I think it’s reasonable to consider it being reimbursed because we’re taking our time and effort and making decisions to help the patient.”
Compensation for portal time affects all physicians, but the degree of impact depends on their specialties. Primary care physicians spent significantly more daily and after-hours time in the EHR, entering notes and orders, and doing clinical reviews compared to surgical and medical specialties.
In addition to the outsized impact on primary care, physician compensation for portal time is also an equity issue.
Dr. Rittenberg researched the issue and found a higher volume of communication from both patients and staff to female physicians than male physicians. As a result, female physicians spend 41.4 minutes more on the EHR than their male counterparts, which equates to more unpaid time. It’s likely no coincidence then that burnout rates are also higher among female physicians, who also leave the clinical workforce in higher numbers, especially in primary care.
“Finding ways to fairly compensate physicians for their work also will address some of the equity issues in workload and the consequences,” Dr. Rittenberg said.
Addressing the Issue
Some health systems have started charging patients who seek medical advice via patient portals, equating the communication to asynchronous acute care or an additional care touchpoint and billing based on the length and complexity of the messages. Patient fees for seeking medical advice via portals vary widely depending on their health system and insurance.
At University of California San Francisco Health, billing patients for EHR communication led to a sharp decrease in patient messages, which eased physician workload. At Cleveland Clinic, physicians receive “productivity credits” for the time spent in the EHR that can be used to reduce their clinic hours (but have no impact on their compensation).
Changes to the Medicare Physician Fee Schedule also allow physicians to bill for “digital evaluation and management” based on the time spent in an EHR responding to patient-initiated questions and requests.
However, more efforts are needed to ease burnout and reverse the number of physicians who are seeing fewer patients or leaving medical practice altogether as a direct result of spending increasing amounts of unpaid time in the EHR. Dr. Rittenberg, who spends an estimated 50% of her working hours in the portal, had to reduce her clinical workload by 25% due to such heavy portal requirements.
“The workload has become unsustainable,” she said. “The work has undergone a dramatic change over the past decade, and the compensation system has not kept up with that change.”
Prioritizing Patient and Physician Experiences
The ever-expanding use of EHR is a result of their value as a healthcare tool. Data showed that the electronic exchange of information between patients and physicians improves diagnostics, reduces medical errors, enhances communication, and leads to more patient-centered care — and physicians want their patients to use the portal to maximize their healthcare.
“[The EHR] is good for patients,” said Dr. DeBiasi. “Sometimes, patients have access issues with healthcare, whether that’s not knowing what number to call or getting the right message to the right person at the right office. If [the portal] is good for them and helps them get access to care, we should embrace that and figure out a way to work it into our day-to-day schedules.”
But maximizing the patient experience shouldn’t come at the physicians’ expense. Dr. Rittenberg advocates a model that compensates physicians for the time spent in the EHR and prioritizes a team approach to rebalance the EHR workload to ensure that physicians aren’t devoting too much time to administrative tasks and can, instead, focus their time on clinical tasks.
“The way in which we provide healthcare has fundamentally shifted, and compensation models need to reflect that new reality,” Dr. Rittenberg added.
A version of this article appeared on Medscape.com.



