User login
Computer-Aided Colonoscopy Falls Short in Real-World Practice
, according to investigators.
Although CADe did not increase burden of colonoscopy in the real-world, these real-world detection rates casts doubt on the generalizability of positive findings from randomized trials, reported lead author Harsh K. Patel, MD, of the University of Kansas Medical Center, Kansas City, Missouri, and colleagues.
CADe-assisted colonoscopy has gained increasing attention for its potential to improve ADR, particularly with the recent publication of a meta-analysis involving 20 randomized controlled trials (RCTs), Dr. Patel and colleagues wrote in Clinical Gastroenterology and Hepatology. “However, results of RCTs are not necessarily reproducible in clinical practice.”
RCTs evaluating this technology are susceptible to various issues with validity, they noted, such as psychological bias stemming from lack of blinding to the possibility that CADe could reduce operator attention, paradoxically “deskilling” endoscopists.
The present meta-analysis aimed to overcome these potential shortfalls by analyzing nonrandomized data from eight studies involving 9,782 patients.
“The lack of a highly controlled setting reduces the psychological pressure of the endoscopists to demonstrate a possible benefit of CADe (i.e., the operator bias) and allows endoscopists to use CADe according to their preferences and attitudes which we usually experience in a real-world clinical practice,” the investigators wrote. “On the other hand, noncontrolled factors may affect the outcome of the study, especially when considering that an equivalent distribution of prevalence of disease is required for a fair assessment of the effectiveness of the intervention.”
This approach revealed less favorable outcomes than those reported by RCTs.
CADe-assisted ADR was not significantly different from ADR for standard colonoscopy (44% vs 38%; risk ratio, 1.11; 95% CI, 0.97-1.28), nor was mean number of adenomas detected per colonoscopy (0.93 vs 0.79; mean difference, 0.14; 95% CI, -0.04-0.32).
“Our study provides a contrasting perspective to those results previously known from the randomized studies,” the investigators wrote.
While detection benefits were not identified, burden of CADe-assisted colonoscopy was not elevated either.
Mean nonneoplastic lesions per colonoscopy was similar between modalities (0.52 vs 0.47; mean difference, 0.14; 95% CI, -0.07-0.34), as was withdrawal time (14.3 vs 13.4 minutes; mean difference, 0.8 minutes; 95% CI, -0.18-1.90).
Dr. Patel and colleagues described “a high level of heterogeneity that was qualitatively and quantitatively distinct from the heterogeneity discovered in the prior meta-analysis of RCTs.” Unlike the RCT meta-analysis, which had no studies with an ADR outcome favoring the control arm, the present meta-analysis found that one third of the included studies favored the control arm.
“This qualitative difference generates a much higher degree of ambiguity, as it does not apply only to the magnitude of the effect of CADe, but it puts in question the actual existence of any CADe-related benefit,” they wrote. “An important point to make is that the analysis of adenoma and serrated lesions per colonoscopy supported the qualitative heterogeneity, favoring the control arm over the CADe arm, in the direction of the effect.”
Dr. Patel and colleagues suggested that the concurrent lack of benefit and lack of harm associated with CADe in the present meta-analysis is “interesting,” and may point to underutilization or a lack of effect of CADe.
“To address the uncertainties in the current literature, we recommend conducting additional randomized studies in a more pragmatic setting,” they concluded.
This meta-analysis was supported by the European Commission and AIRC. The investigators disclosed relationships with NEC, Satisfy, Odin, and others.
The advent of AI in colonoscopy through computer-aided detection (CADe) systems has been promising, with over 20 randomized controlled trials (RCTs) affirming its benefits. However, this enthusiasm has been tempered by several recent nonrandomized studies indicating no real-world advantage, as discussed in Patel et al.’s systematic review and meta-analysis in Clinical Gastroenterology and Hepatology.
In addition, a critical consideration with evaluating any AI/CADe system is they often undergo frequent updates, each promising improved accuracy, sensitivity, and specificity. This is an interesting dilemma and raises questions about the enduring relevance of studies conducted using outdated versions of CADe.
In my opinion, the jury is still out on the effectiveness of CADe for colonoscopy in a real-world setting. The definitive assessment of CADe’s real-world value necessitates larger, well-structured trials that mirror actual clinical environments and span extended periods of time, taking care to minimize biases that may have influenced the results of current published studies.
Nabil M. Mansour, MD, is assistant professor of medicine in the Section of Gastroenterology, Baylor College of Medicine, Houston. He has served as a consultant for Iterative Health.
The advent of AI in colonoscopy through computer-aided detection (CADe) systems has been promising, with over 20 randomized controlled trials (RCTs) affirming its benefits. However, this enthusiasm has been tempered by several recent nonrandomized studies indicating no real-world advantage, as discussed in Patel et al.’s systematic review and meta-analysis in Clinical Gastroenterology and Hepatology.
In addition, a critical consideration with evaluating any AI/CADe system is they often undergo frequent updates, each promising improved accuracy, sensitivity, and specificity. This is an interesting dilemma and raises questions about the enduring relevance of studies conducted using outdated versions of CADe.
In my opinion, the jury is still out on the effectiveness of CADe for colonoscopy in a real-world setting. The definitive assessment of CADe’s real-world value necessitates larger, well-structured trials that mirror actual clinical environments and span extended periods of time, taking care to minimize biases that may have influenced the results of current published studies.
Nabil M. Mansour, MD, is assistant professor of medicine in the Section of Gastroenterology, Baylor College of Medicine, Houston. He has served as a consultant for Iterative Health.
The advent of AI in colonoscopy through computer-aided detection (CADe) systems has been promising, with over 20 randomized controlled trials (RCTs) affirming its benefits. However, this enthusiasm has been tempered by several recent nonrandomized studies indicating no real-world advantage, as discussed in Patel et al.’s systematic review and meta-analysis in Clinical Gastroenterology and Hepatology.
In addition, a critical consideration with evaluating any AI/CADe system is they often undergo frequent updates, each promising improved accuracy, sensitivity, and specificity. This is an interesting dilemma and raises questions about the enduring relevance of studies conducted using outdated versions of CADe.
In my opinion, the jury is still out on the effectiveness of CADe for colonoscopy in a real-world setting. The definitive assessment of CADe’s real-world value necessitates larger, well-structured trials that mirror actual clinical environments and span extended periods of time, taking care to minimize biases that may have influenced the results of current published studies.
Nabil M. Mansour, MD, is assistant professor of medicine in the Section of Gastroenterology, Baylor College of Medicine, Houston. He has served as a consultant for Iterative Health.
, according to investigators.
Although CADe did not increase burden of colonoscopy in the real-world, these real-world detection rates casts doubt on the generalizability of positive findings from randomized trials, reported lead author Harsh K. Patel, MD, of the University of Kansas Medical Center, Kansas City, Missouri, and colleagues.
CADe-assisted colonoscopy has gained increasing attention for its potential to improve ADR, particularly with the recent publication of a meta-analysis involving 20 randomized controlled trials (RCTs), Dr. Patel and colleagues wrote in Clinical Gastroenterology and Hepatology. “However, results of RCTs are not necessarily reproducible in clinical practice.”
RCTs evaluating this technology are susceptible to various issues with validity, they noted, such as psychological bias stemming from lack of blinding to the possibility that CADe could reduce operator attention, paradoxically “deskilling” endoscopists.
The present meta-analysis aimed to overcome these potential shortfalls by analyzing nonrandomized data from eight studies involving 9,782 patients.
“The lack of a highly controlled setting reduces the psychological pressure of the endoscopists to demonstrate a possible benefit of CADe (i.e., the operator bias) and allows endoscopists to use CADe according to their preferences and attitudes which we usually experience in a real-world clinical practice,” the investigators wrote. “On the other hand, noncontrolled factors may affect the outcome of the study, especially when considering that an equivalent distribution of prevalence of disease is required for a fair assessment of the effectiveness of the intervention.”
This approach revealed less favorable outcomes than those reported by RCTs.
CADe-assisted ADR was not significantly different from ADR for standard colonoscopy (44% vs 38%; risk ratio, 1.11; 95% CI, 0.97-1.28), nor was mean number of adenomas detected per colonoscopy (0.93 vs 0.79; mean difference, 0.14; 95% CI, -0.04-0.32).
“Our study provides a contrasting perspective to those results previously known from the randomized studies,” the investigators wrote.
While detection benefits were not identified, burden of CADe-assisted colonoscopy was not elevated either.
Mean nonneoplastic lesions per colonoscopy was similar between modalities (0.52 vs 0.47; mean difference, 0.14; 95% CI, -0.07-0.34), as was withdrawal time (14.3 vs 13.4 minutes; mean difference, 0.8 minutes; 95% CI, -0.18-1.90).
Dr. Patel and colleagues described “a high level of heterogeneity that was qualitatively and quantitatively distinct from the heterogeneity discovered in the prior meta-analysis of RCTs.” Unlike the RCT meta-analysis, which had no studies with an ADR outcome favoring the control arm, the present meta-analysis found that one third of the included studies favored the control arm.
“This qualitative difference generates a much higher degree of ambiguity, as it does not apply only to the magnitude of the effect of CADe, but it puts in question the actual existence of any CADe-related benefit,” they wrote. “An important point to make is that the analysis of adenoma and serrated lesions per colonoscopy supported the qualitative heterogeneity, favoring the control arm over the CADe arm, in the direction of the effect.”
Dr. Patel and colleagues suggested that the concurrent lack of benefit and lack of harm associated with CADe in the present meta-analysis is “interesting,” and may point to underutilization or a lack of effect of CADe.
“To address the uncertainties in the current literature, we recommend conducting additional randomized studies in a more pragmatic setting,” they concluded.
This meta-analysis was supported by the European Commission and AIRC. The investigators disclosed relationships with NEC, Satisfy, Odin, and others.
, according to investigators.
Although CADe did not increase burden of colonoscopy in the real-world, these real-world detection rates casts doubt on the generalizability of positive findings from randomized trials, reported lead author Harsh K. Patel, MD, of the University of Kansas Medical Center, Kansas City, Missouri, and colleagues.
CADe-assisted colonoscopy has gained increasing attention for its potential to improve ADR, particularly with the recent publication of a meta-analysis involving 20 randomized controlled trials (RCTs), Dr. Patel and colleagues wrote in Clinical Gastroenterology and Hepatology. “However, results of RCTs are not necessarily reproducible in clinical practice.”
RCTs evaluating this technology are susceptible to various issues with validity, they noted, such as psychological bias stemming from lack of blinding to the possibility that CADe could reduce operator attention, paradoxically “deskilling” endoscopists.
The present meta-analysis aimed to overcome these potential shortfalls by analyzing nonrandomized data from eight studies involving 9,782 patients.
“The lack of a highly controlled setting reduces the psychological pressure of the endoscopists to demonstrate a possible benefit of CADe (i.e., the operator bias) and allows endoscopists to use CADe according to their preferences and attitudes which we usually experience in a real-world clinical practice,” the investigators wrote. “On the other hand, noncontrolled factors may affect the outcome of the study, especially when considering that an equivalent distribution of prevalence of disease is required for a fair assessment of the effectiveness of the intervention.”
This approach revealed less favorable outcomes than those reported by RCTs.
CADe-assisted ADR was not significantly different from ADR for standard colonoscopy (44% vs 38%; risk ratio, 1.11; 95% CI, 0.97-1.28), nor was mean number of adenomas detected per colonoscopy (0.93 vs 0.79; mean difference, 0.14; 95% CI, -0.04-0.32).
“Our study provides a contrasting perspective to those results previously known from the randomized studies,” the investigators wrote.
While detection benefits were not identified, burden of CADe-assisted colonoscopy was not elevated either.
Mean nonneoplastic lesions per colonoscopy was similar between modalities (0.52 vs 0.47; mean difference, 0.14; 95% CI, -0.07-0.34), as was withdrawal time (14.3 vs 13.4 minutes; mean difference, 0.8 minutes; 95% CI, -0.18-1.90).
Dr. Patel and colleagues described “a high level of heterogeneity that was qualitatively and quantitatively distinct from the heterogeneity discovered in the prior meta-analysis of RCTs.” Unlike the RCT meta-analysis, which had no studies with an ADR outcome favoring the control arm, the present meta-analysis found that one third of the included studies favored the control arm.
“This qualitative difference generates a much higher degree of ambiguity, as it does not apply only to the magnitude of the effect of CADe, but it puts in question the actual existence of any CADe-related benefit,” they wrote. “An important point to make is that the analysis of adenoma and serrated lesions per colonoscopy supported the qualitative heterogeneity, favoring the control arm over the CADe arm, in the direction of the effect.”
Dr. Patel and colleagues suggested that the concurrent lack of benefit and lack of harm associated with CADe in the present meta-analysis is “interesting,” and may point to underutilization or a lack of effect of CADe.
“To address the uncertainties in the current literature, we recommend conducting additional randomized studies in a more pragmatic setting,” they concluded.
This meta-analysis was supported by the European Commission and AIRC. The investigators disclosed relationships with NEC, Satisfy, Odin, and others.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
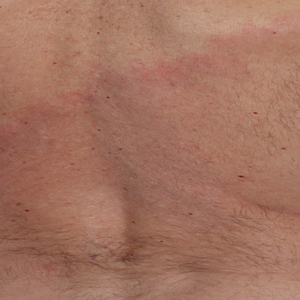
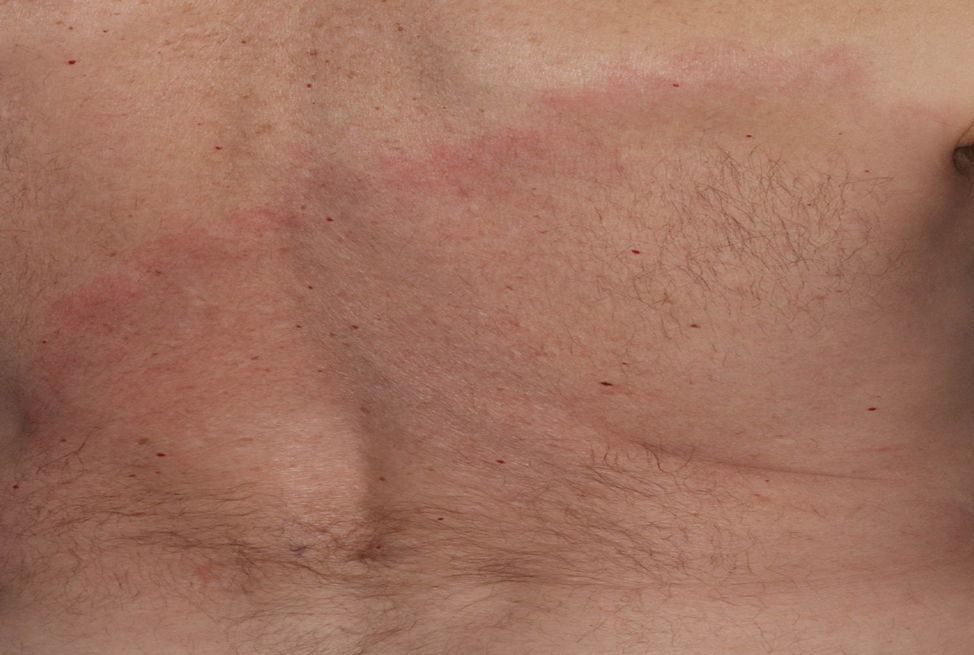
Asymptomatic Erythematous Plaque in an Outdoorsman
The Diagnosis: Erythema Migrans
The patient was clinically diagnosed with erythema migrans. He did not recall a tick bite but spent a lot of time outdoors. He was treated with 10 days of doxycycline 100 mg twice daily with complete resolution of the rash.
Lyme disease is a spirochete infection caused by the Borrelia burgdorferi sensu lato species complex and transmitted by the Ixodidae tick family. It is the most common tick-borne disease in the United States and mostly is reported in the northeastern and upper midwestern states during the warmer seasons, but it is prevalent worldwide. In geographic areas where Lyme disease is common, the incidence is approximately 40 cases per 100,000 individuals.1 Our patient resided in coastal South Carolina. Lyme disease is more commonly reported in White individuals. The skin lesions may be more difficult to discern and diagnose in patients with darker skin types, leading to delayed diagnosis and treatment.2,3
Patients may be diagnosed with early localized, early disseminated, or late Lyme disease. Erythema migrans is the early localized form of the disease and is classically described as an erythematous targetlike plaque with raised borders arising at the site of the tick bite 1 to 2 weeks later.4 However, many patients simply have a homogeneous erythematous plaque with raised advancing borders ranging in size from 5 to 68 cm.5 In a 2022 study of 69 patients with suspected Lyme disease, only 35 (50.7%) were determined to truly have acute Lyme disease.6 Of them, only 2 (5.7%) had the classic ringwithin- a-ring pattern. Most plaques were uniform, pink, oval-shaped lesions with well-demarcated borders.6
The rash may present with a burning sensation, or patients may experience no symptoms at all, which can lead to delayed diagnosis and progression to late disease. Patients may develop malaise, fever, headache, body aches, or joint pain. Early disseminated disease manifests similarly. Patients with disseminated disease also may develop more serious complications, including lymphadenopathy; cranial nerve palsies; ocular involvement; meningitis; or cardiac abnormalities such as myocarditis, pericarditis, or arrhythmia. Late disease most often causes arthritis of the large joints, though it also can have cardiac or neurologic manifestations. Some patients with chronic disease—the majority of whom were diagnosed in Europe—may develop acrodermatitis chronica atrophicans with edematous blue-red plaques that become atrophic and hyperpigmented fibrotic plaques over the course of years.
Allergic contact dermatitis to a plant more likely would cause itchy or painful, oozy, weepy, vesicular lesions arranged in a linear pattern. A dermatophyte infection likely would cause a scaly eruption. Although our patient presented with a sharply demarcated, raised, erythematous lesion, the distribution did not follow normal clothing lines and would be unusual for a photosensitive drug eruption. Cellulitis likely would be associated with tenderness or warmth to the touch. Finally, southern tick-associated rash illness, which is associated with Amblyomma americanum (lone star tick) bites, may appear with a similar rash but few systemic symptoms. It also can be treated with tetracycline antibiotics.7
Our case in South Carolina demonstrates the importance of keeping Lyme disease in the differential. Clinicians should remember to ask patients about their travel history. In endemic areas, patients with erythema migrans can be started on treatment without waiting for serology. Patients with early Lyme disease may or may not have positive serologies at the time of presentation.6 Guidelines for the treatment of Lyme disease have been revised in recent years to decrease patient antibiotic exposure by reducing the number of days of antibiotic therapy.8 A recent randomized controlled trial found no significant difference in recurrence for patients treated with 7 days of doxycycline compared with 14 days.9 We typically prescribe a 10-day course of doxycycline, which also is adequate for concurrent rickettsial disease. Patients who develop malarialike symptoms should be evaluated for babesiosis, which is treated with clindamycin.
- Skar GL, Simonsen KA. Lyme disease. StatPearls [Internet]. Updated February 4, 2024. Accessed March 20, 2024. https://www.ncbi.nlm.nih.gov/books/NBK431066/
- Dennison R, Novak C, Rebman A, et al. Lyme disease with erythema migrans and seventh nerve palsy in an African-American man. Cureus. 2019;11:E6509.
- Bax CE, Clark AK, Oboite M, et al. A case of disseminated Lyme disease in a child with skin of color. Pediatr Dermatol. 2021;38 (suppl 2):140-141.
- Shah AS, Varatharaj Palraj BR. Multiple erythema migrans rashes characteristic of early disseminated lyme disease, before and after therapy. Mayo Clin Proc. 2019;94:172-173.
- Feder HM Jr, Abeles M, Bernstein M, et al. Diagnosis, treatment, and prognosis of erythema migrans and Lyme arthritis. Clin Dermatol. 2006;24:509-520.
- Schotthoefer AM, Green CB, Dempsey G, et al. The spectrum of erythema migrans in early Lyme disease: can we improve its recognition? Cureus. 2022;14:E30673.
- Strle F, Wormser GP. Early Lyme disease (erythema migrans) and its mimics (southern tick-associated rash illness and tick-associated rash illness). Infect Dis Clin North Am. 2022;36:523-539.
- Torbahn G, Hofmann H, Rücker G, et al. Efficacy and safety of antibiotic therapy in early cutaneous Lyme borreliosis: a network meta-analysis. JAMA Dermatol. 2018;154:1292-1303.
- Stupica D, Collinet-Adler S, Blagus R, et al. Treatment of erythema migrans with doxycycline for 7 days versus 14 days in Slovenia: a randomised open-label non-inferiority trial. Lancet Infect Dis. 2023;23:371-379.
The Diagnosis: Erythema Migrans
The patient was clinically diagnosed with erythema migrans. He did not recall a tick bite but spent a lot of time outdoors. He was treated with 10 days of doxycycline 100 mg twice daily with complete resolution of the rash.
Lyme disease is a spirochete infection caused by the Borrelia burgdorferi sensu lato species complex and transmitted by the Ixodidae tick family. It is the most common tick-borne disease in the United States and mostly is reported in the northeastern and upper midwestern states during the warmer seasons, but it is prevalent worldwide. In geographic areas where Lyme disease is common, the incidence is approximately 40 cases per 100,000 individuals.1 Our patient resided in coastal South Carolina. Lyme disease is more commonly reported in White individuals. The skin lesions may be more difficult to discern and diagnose in patients with darker skin types, leading to delayed diagnosis and treatment.2,3
Patients may be diagnosed with early localized, early disseminated, or late Lyme disease. Erythema migrans is the early localized form of the disease and is classically described as an erythematous targetlike plaque with raised borders arising at the site of the tick bite 1 to 2 weeks later.4 However, many patients simply have a homogeneous erythematous plaque with raised advancing borders ranging in size from 5 to 68 cm.5 In a 2022 study of 69 patients with suspected Lyme disease, only 35 (50.7%) were determined to truly have acute Lyme disease.6 Of them, only 2 (5.7%) had the classic ringwithin- a-ring pattern. Most plaques were uniform, pink, oval-shaped lesions with well-demarcated borders.6
The rash may present with a burning sensation, or patients may experience no symptoms at all, which can lead to delayed diagnosis and progression to late disease. Patients may develop malaise, fever, headache, body aches, or joint pain. Early disseminated disease manifests similarly. Patients with disseminated disease also may develop more serious complications, including lymphadenopathy; cranial nerve palsies; ocular involvement; meningitis; or cardiac abnormalities such as myocarditis, pericarditis, or arrhythmia. Late disease most often causes arthritis of the large joints, though it also can have cardiac or neurologic manifestations. Some patients with chronic disease—the majority of whom were diagnosed in Europe—may develop acrodermatitis chronica atrophicans with edematous blue-red plaques that become atrophic and hyperpigmented fibrotic plaques over the course of years.
Allergic contact dermatitis to a plant more likely would cause itchy or painful, oozy, weepy, vesicular lesions arranged in a linear pattern. A dermatophyte infection likely would cause a scaly eruption. Although our patient presented with a sharply demarcated, raised, erythematous lesion, the distribution did not follow normal clothing lines and would be unusual for a photosensitive drug eruption. Cellulitis likely would be associated with tenderness or warmth to the touch. Finally, southern tick-associated rash illness, which is associated with Amblyomma americanum (lone star tick) bites, may appear with a similar rash but few systemic symptoms. It also can be treated with tetracycline antibiotics.7
Our case in South Carolina demonstrates the importance of keeping Lyme disease in the differential. Clinicians should remember to ask patients about their travel history. In endemic areas, patients with erythema migrans can be started on treatment without waiting for serology. Patients with early Lyme disease may or may not have positive serologies at the time of presentation.6 Guidelines for the treatment of Lyme disease have been revised in recent years to decrease patient antibiotic exposure by reducing the number of days of antibiotic therapy.8 A recent randomized controlled trial found no significant difference in recurrence for patients treated with 7 days of doxycycline compared with 14 days.9 We typically prescribe a 10-day course of doxycycline, which also is adequate for concurrent rickettsial disease. Patients who develop malarialike symptoms should be evaluated for babesiosis, which is treated with clindamycin.
The Diagnosis: Erythema Migrans
The patient was clinically diagnosed with erythema migrans. He did not recall a tick bite but spent a lot of time outdoors. He was treated with 10 days of doxycycline 100 mg twice daily with complete resolution of the rash.
Lyme disease is a spirochete infection caused by the Borrelia burgdorferi sensu lato species complex and transmitted by the Ixodidae tick family. It is the most common tick-borne disease in the United States and mostly is reported in the northeastern and upper midwestern states during the warmer seasons, but it is prevalent worldwide. In geographic areas where Lyme disease is common, the incidence is approximately 40 cases per 100,000 individuals.1 Our patient resided in coastal South Carolina. Lyme disease is more commonly reported in White individuals. The skin lesions may be more difficult to discern and diagnose in patients with darker skin types, leading to delayed diagnosis and treatment.2,3
Patients may be diagnosed with early localized, early disseminated, or late Lyme disease. Erythema migrans is the early localized form of the disease and is classically described as an erythematous targetlike plaque with raised borders arising at the site of the tick bite 1 to 2 weeks later.4 However, many patients simply have a homogeneous erythematous plaque with raised advancing borders ranging in size from 5 to 68 cm.5 In a 2022 study of 69 patients with suspected Lyme disease, only 35 (50.7%) were determined to truly have acute Lyme disease.6 Of them, only 2 (5.7%) had the classic ringwithin- a-ring pattern. Most plaques were uniform, pink, oval-shaped lesions with well-demarcated borders.6
The rash may present with a burning sensation, or patients may experience no symptoms at all, which can lead to delayed diagnosis and progression to late disease. Patients may develop malaise, fever, headache, body aches, or joint pain. Early disseminated disease manifests similarly. Patients with disseminated disease also may develop more serious complications, including lymphadenopathy; cranial nerve palsies; ocular involvement; meningitis; or cardiac abnormalities such as myocarditis, pericarditis, or arrhythmia. Late disease most often causes arthritis of the large joints, though it also can have cardiac or neurologic manifestations. Some patients with chronic disease—the majority of whom were diagnosed in Europe—may develop acrodermatitis chronica atrophicans with edematous blue-red plaques that become atrophic and hyperpigmented fibrotic plaques over the course of years.
Allergic contact dermatitis to a plant more likely would cause itchy or painful, oozy, weepy, vesicular lesions arranged in a linear pattern. A dermatophyte infection likely would cause a scaly eruption. Although our patient presented with a sharply demarcated, raised, erythematous lesion, the distribution did not follow normal clothing lines and would be unusual for a photosensitive drug eruption. Cellulitis likely would be associated with tenderness or warmth to the touch. Finally, southern tick-associated rash illness, which is associated with Amblyomma americanum (lone star tick) bites, may appear with a similar rash but few systemic symptoms. It also can be treated with tetracycline antibiotics.7
Our case in South Carolina demonstrates the importance of keeping Lyme disease in the differential. Clinicians should remember to ask patients about their travel history. In endemic areas, patients with erythema migrans can be started on treatment without waiting for serology. Patients with early Lyme disease may or may not have positive serologies at the time of presentation.6 Guidelines for the treatment of Lyme disease have been revised in recent years to decrease patient antibiotic exposure by reducing the number of days of antibiotic therapy.8 A recent randomized controlled trial found no significant difference in recurrence for patients treated with 7 days of doxycycline compared with 14 days.9 We typically prescribe a 10-day course of doxycycline, which also is adequate for concurrent rickettsial disease. Patients who develop malarialike symptoms should be evaluated for babesiosis, which is treated with clindamycin.
- Skar GL, Simonsen KA. Lyme disease. StatPearls [Internet]. Updated February 4, 2024. Accessed March 20, 2024. https://www.ncbi.nlm.nih.gov/books/NBK431066/
- Dennison R, Novak C, Rebman A, et al. Lyme disease with erythema migrans and seventh nerve palsy in an African-American man. Cureus. 2019;11:E6509.
- Bax CE, Clark AK, Oboite M, et al. A case of disseminated Lyme disease in a child with skin of color. Pediatr Dermatol. 2021;38 (suppl 2):140-141.
- Shah AS, Varatharaj Palraj BR. Multiple erythema migrans rashes characteristic of early disseminated lyme disease, before and after therapy. Mayo Clin Proc. 2019;94:172-173.
- Feder HM Jr, Abeles M, Bernstein M, et al. Diagnosis, treatment, and prognosis of erythema migrans and Lyme arthritis. Clin Dermatol. 2006;24:509-520.
- Schotthoefer AM, Green CB, Dempsey G, et al. The spectrum of erythema migrans in early Lyme disease: can we improve its recognition? Cureus. 2022;14:E30673.
- Strle F, Wormser GP. Early Lyme disease (erythema migrans) and its mimics (southern tick-associated rash illness and tick-associated rash illness). Infect Dis Clin North Am. 2022;36:523-539.
- Torbahn G, Hofmann H, Rücker G, et al. Efficacy and safety of antibiotic therapy in early cutaneous Lyme borreliosis: a network meta-analysis. JAMA Dermatol. 2018;154:1292-1303.
- Stupica D, Collinet-Adler S, Blagus R, et al. Treatment of erythema migrans with doxycycline for 7 days versus 14 days in Slovenia: a randomised open-label non-inferiority trial. Lancet Infect Dis. 2023;23:371-379.
- Skar GL, Simonsen KA. Lyme disease. StatPearls [Internet]. Updated February 4, 2024. Accessed March 20, 2024. https://www.ncbi.nlm.nih.gov/books/NBK431066/
- Dennison R, Novak C, Rebman A, et al. Lyme disease with erythema migrans and seventh nerve palsy in an African-American man. Cureus. 2019;11:E6509.
- Bax CE, Clark AK, Oboite M, et al. A case of disseminated Lyme disease in a child with skin of color. Pediatr Dermatol. 2021;38 (suppl 2):140-141.
- Shah AS, Varatharaj Palraj BR. Multiple erythema migrans rashes characteristic of early disseminated lyme disease, before and after therapy. Mayo Clin Proc. 2019;94:172-173.
- Feder HM Jr, Abeles M, Bernstein M, et al. Diagnosis, treatment, and prognosis of erythema migrans and Lyme arthritis. Clin Dermatol. 2006;24:509-520.
- Schotthoefer AM, Green CB, Dempsey G, et al. The spectrum of erythema migrans in early Lyme disease: can we improve its recognition? Cureus. 2022;14:E30673.
- Strle F, Wormser GP. Early Lyme disease (erythema migrans) and its mimics (southern tick-associated rash illness and tick-associated rash illness). Infect Dis Clin North Am. 2022;36:523-539.
- Torbahn G, Hofmann H, Rücker G, et al. Efficacy and safety of antibiotic therapy in early cutaneous Lyme borreliosis: a network meta-analysis. JAMA Dermatol. 2018;154:1292-1303.
- Stupica D, Collinet-Adler S, Blagus R, et al. Treatment of erythema migrans with doxycycline for 7 days versus 14 days in Slovenia: a randomised open-label non-inferiority trial. Lancet Infect Dis. 2023;23:371-379.
A middle-aged man presented with a well-demarcated, hyperpigmented, erythematous patch with an annular erythematous border that extended from the mid-back to the lower back. The patient was otherwise asymptomatic. He was an avid gardener who resided in South Carolina and had recently adopted 2 puppies.
Think Beyond the ‘Go-Tos’ for Wart Management, Expert Advises
SAN DIEGO — When Jennifer Adams, MD, recently entered the search term “warts” on the ClinicalTrials.gov web site, nearly 240 results popped up.
“There is a lot of research activity around this topic,” Dr. Adams, vice chair of the department of dermatology at the University of Nebraska Medical Center, said at the annual meeting of the American Academy of Dermatology. “We just don’t have fantastic, well-run trials on many of the currently available treatments.”
In a 2012 Cochrane review on the topical treatment of non-genital cutaneous warts, authors drew from 85 trials involving 8,815 randomized patients. They found that most warts spontaneously resolved, and the authors determined salicylic acid to be safe and modestly beneficial. Specifically, trials of salicylic acid (SA) versus placebo showed that the former significantly increased the chance of clearance of warts at all sites (risk ratio, 1.56, 95% confidence interval [CI], 1.20-2.03). A meta-analysis of cryotherapy versus placebo for warts at all sites favored neither intervention nor control (RR, 1.45, 95% CI, 0.65-3.23).
“The authors determined that there is less evidence for cryotherapy but stated that it may work when salicylic acid does not, or in combination with salicylic acid,” Dr. Adams said. “However, salicylic acid and cryotherapy don’t do enough for our patients [with warts]. There are a lot of situations where we need to reach further into the toolbox.”
A 2021 review article listed many options for managing difficult-to-treat warts, including intralesional Candida antigen, intralesional measles-mumps-rubella (MMR), intralesional HPV vaccine, intralesional vitamin D, intralesional cidofovir, intralesional bleomycin, and intralesional 5-FU injections, and topical vitamin D, topical cidofovir, and topical bleomycin. According to Dr. Adams, clinical data exist for cidofovir and vitamin D but studies evaluated different formulations, doses, sites of administration, and limited randomized controlled trials.
“Intralesional cidofovir is more effective than the topical form, but intralesional cidofovir can be painful and both forms are expensive,” she said. “Topical vitamin D is less likely to cause dyspigmentation compared to other available treatments, so it’s a great option in skin of color, but it has been less effective compared to some of our other topical treatments.”
Newer Options Promising
On the horizon, berdazimer gel was approved in January of 2024 for the treatment of molluscum but results from trials of its use for extragenital warts are encouraging. Another promising option is topical ionic contraviral therapy (ICVT) with digoxin and furosemide combined, which inhibits cellular potassium influx. A phase 2a randomized controlled trial of 80 adults found a statistically significant reduction in the diameter of cutaneous warts among those who received ICVT compared with those who received placebo (P = .002). “It’s cheap and well tolerated,” Dr. Adams added.
Intralesional approaches to treating warts offer another alternative. A 2020 review of 43 articles concluded that intralesional treatments for warts have equal or superior efficacy to first-line salicylic acid or cryotherapy.
Dr. Adams said that she considers intralesional treatments such as vitamin D, MMR vaccine antigen, and Candida antigen for refractory, numerous, or distant site warts. “Injecting the MMR vaccine into the largest wart every 2 weeks has been found to lead to complete clearance in 60%-68% of cases in one study,” she said. “The benefit is that it’s $21 per dose, which is nice, but as with any vaccination, patients can develop flu-like symptoms as side effects.”
Use of the HPV vaccine for treating cutaneous warts remains controversial, she continued, but it seems to work better in younger patients. In one open-label study that evaluated the HPV vaccine for the treatment of multiple recalcitrant warts, with doses administered at 0. 2, and 6 months, the response rate 3 months after the third dose was 55% among those older than age 26, compared with 84% among those ages 9-26 years.
Another option, intralesional cidofovir, has been shown to be especially effective for refractory warts. “It has also been shown to work for warts in immunocompetent and immunocompromised patients,” Dr. Adams said.
In the realm of adjuvant treatments, microneedling has been found to have similar efficacy to needling, Dr. Adams said, but with minimal pain. “When we combine it with topical treatments like 5-FU, it’s even more efficacious,” she said.
One study found that combining microneedling with topical 5-FU had clearance similar to that of intralesional 5-FU or microneedling alone, but involved fewer treatment sessions and less pain in the combination group.
Autoinoculation has been used to stimulate an immune response in patients with warts, leading to clearance rates of 4% (mild clearance) to 66% (complete clearance) in one study. “We would expect this to work better in immunocompetent patients, but it’s something to keep in mind if you’re limited in the medications you can get for a patient,” Dr. Adams said. Also, results from a systematic review and meta-analysis suggest that systemic retinoids combined with intralesional immunotherapy leads to higher clearance rates and lower rates of recurrence of warts. The top performer among those tested was acitretin plus Candida antigen.
Dr. Adams advised dermatologists who try alternatives to salicylic acid and cryotherapy for warts to be “wary of a lack of high-level evidence” for their use. “They can be helpful for patients who have failed traditional therapies or have a contraindication to the usual go-tos.”
She reported having no relevant financial disclosures.
SAN DIEGO — When Jennifer Adams, MD, recently entered the search term “warts” on the ClinicalTrials.gov web site, nearly 240 results popped up.
“There is a lot of research activity around this topic,” Dr. Adams, vice chair of the department of dermatology at the University of Nebraska Medical Center, said at the annual meeting of the American Academy of Dermatology. “We just don’t have fantastic, well-run trials on many of the currently available treatments.”
In a 2012 Cochrane review on the topical treatment of non-genital cutaneous warts, authors drew from 85 trials involving 8,815 randomized patients. They found that most warts spontaneously resolved, and the authors determined salicylic acid to be safe and modestly beneficial. Specifically, trials of salicylic acid (SA) versus placebo showed that the former significantly increased the chance of clearance of warts at all sites (risk ratio, 1.56, 95% confidence interval [CI], 1.20-2.03). A meta-analysis of cryotherapy versus placebo for warts at all sites favored neither intervention nor control (RR, 1.45, 95% CI, 0.65-3.23).
“The authors determined that there is less evidence for cryotherapy but stated that it may work when salicylic acid does not, or in combination with salicylic acid,” Dr. Adams said. “However, salicylic acid and cryotherapy don’t do enough for our patients [with warts]. There are a lot of situations where we need to reach further into the toolbox.”
A 2021 review article listed many options for managing difficult-to-treat warts, including intralesional Candida antigen, intralesional measles-mumps-rubella (MMR), intralesional HPV vaccine, intralesional vitamin D, intralesional cidofovir, intralesional bleomycin, and intralesional 5-FU injections, and topical vitamin D, topical cidofovir, and topical bleomycin. According to Dr. Adams, clinical data exist for cidofovir and vitamin D but studies evaluated different formulations, doses, sites of administration, and limited randomized controlled trials.
“Intralesional cidofovir is more effective than the topical form, but intralesional cidofovir can be painful and both forms are expensive,” she said. “Topical vitamin D is less likely to cause dyspigmentation compared to other available treatments, so it’s a great option in skin of color, but it has been less effective compared to some of our other topical treatments.”
Newer Options Promising
On the horizon, berdazimer gel was approved in January of 2024 for the treatment of molluscum but results from trials of its use for extragenital warts are encouraging. Another promising option is topical ionic contraviral therapy (ICVT) with digoxin and furosemide combined, which inhibits cellular potassium influx. A phase 2a randomized controlled trial of 80 adults found a statistically significant reduction in the diameter of cutaneous warts among those who received ICVT compared with those who received placebo (P = .002). “It’s cheap and well tolerated,” Dr. Adams added.
Intralesional approaches to treating warts offer another alternative. A 2020 review of 43 articles concluded that intralesional treatments for warts have equal or superior efficacy to first-line salicylic acid or cryotherapy.
Dr. Adams said that she considers intralesional treatments such as vitamin D, MMR vaccine antigen, and Candida antigen for refractory, numerous, or distant site warts. “Injecting the MMR vaccine into the largest wart every 2 weeks has been found to lead to complete clearance in 60%-68% of cases in one study,” she said. “The benefit is that it’s $21 per dose, which is nice, but as with any vaccination, patients can develop flu-like symptoms as side effects.”
Use of the HPV vaccine for treating cutaneous warts remains controversial, she continued, but it seems to work better in younger patients. In one open-label study that evaluated the HPV vaccine for the treatment of multiple recalcitrant warts, with doses administered at 0. 2, and 6 months, the response rate 3 months after the third dose was 55% among those older than age 26, compared with 84% among those ages 9-26 years.
Another option, intralesional cidofovir, has been shown to be especially effective for refractory warts. “It has also been shown to work for warts in immunocompetent and immunocompromised patients,” Dr. Adams said.
In the realm of adjuvant treatments, microneedling has been found to have similar efficacy to needling, Dr. Adams said, but with minimal pain. “When we combine it with topical treatments like 5-FU, it’s even more efficacious,” she said.
One study found that combining microneedling with topical 5-FU had clearance similar to that of intralesional 5-FU or microneedling alone, but involved fewer treatment sessions and less pain in the combination group.
Autoinoculation has been used to stimulate an immune response in patients with warts, leading to clearance rates of 4% (mild clearance) to 66% (complete clearance) in one study. “We would expect this to work better in immunocompetent patients, but it’s something to keep in mind if you’re limited in the medications you can get for a patient,” Dr. Adams said. Also, results from a systematic review and meta-analysis suggest that systemic retinoids combined with intralesional immunotherapy leads to higher clearance rates and lower rates of recurrence of warts. The top performer among those tested was acitretin plus Candida antigen.
Dr. Adams advised dermatologists who try alternatives to salicylic acid and cryotherapy for warts to be “wary of a lack of high-level evidence” for their use. “They can be helpful for patients who have failed traditional therapies or have a contraindication to the usual go-tos.”
She reported having no relevant financial disclosures.
SAN DIEGO — When Jennifer Adams, MD, recently entered the search term “warts” on the ClinicalTrials.gov web site, nearly 240 results popped up.
“There is a lot of research activity around this topic,” Dr. Adams, vice chair of the department of dermatology at the University of Nebraska Medical Center, said at the annual meeting of the American Academy of Dermatology. “We just don’t have fantastic, well-run trials on many of the currently available treatments.”
In a 2012 Cochrane review on the topical treatment of non-genital cutaneous warts, authors drew from 85 trials involving 8,815 randomized patients. They found that most warts spontaneously resolved, and the authors determined salicylic acid to be safe and modestly beneficial. Specifically, trials of salicylic acid (SA) versus placebo showed that the former significantly increased the chance of clearance of warts at all sites (risk ratio, 1.56, 95% confidence interval [CI], 1.20-2.03). A meta-analysis of cryotherapy versus placebo for warts at all sites favored neither intervention nor control (RR, 1.45, 95% CI, 0.65-3.23).
“The authors determined that there is less evidence for cryotherapy but stated that it may work when salicylic acid does not, or in combination with salicylic acid,” Dr. Adams said. “However, salicylic acid and cryotherapy don’t do enough for our patients [with warts]. There are a lot of situations where we need to reach further into the toolbox.”
A 2021 review article listed many options for managing difficult-to-treat warts, including intralesional Candida antigen, intralesional measles-mumps-rubella (MMR), intralesional HPV vaccine, intralesional vitamin D, intralesional cidofovir, intralesional bleomycin, and intralesional 5-FU injections, and topical vitamin D, topical cidofovir, and topical bleomycin. According to Dr. Adams, clinical data exist for cidofovir and vitamin D but studies evaluated different formulations, doses, sites of administration, and limited randomized controlled trials.
“Intralesional cidofovir is more effective than the topical form, but intralesional cidofovir can be painful and both forms are expensive,” she said. “Topical vitamin D is less likely to cause dyspigmentation compared to other available treatments, so it’s a great option in skin of color, but it has been less effective compared to some of our other topical treatments.”
Newer Options Promising
On the horizon, berdazimer gel was approved in January of 2024 for the treatment of molluscum but results from trials of its use for extragenital warts are encouraging. Another promising option is topical ionic contraviral therapy (ICVT) with digoxin and furosemide combined, which inhibits cellular potassium influx. A phase 2a randomized controlled trial of 80 adults found a statistically significant reduction in the diameter of cutaneous warts among those who received ICVT compared with those who received placebo (P = .002). “It’s cheap and well tolerated,” Dr. Adams added.
Intralesional approaches to treating warts offer another alternative. A 2020 review of 43 articles concluded that intralesional treatments for warts have equal or superior efficacy to first-line salicylic acid or cryotherapy.
Dr. Adams said that she considers intralesional treatments such as vitamin D, MMR vaccine antigen, and Candida antigen for refractory, numerous, or distant site warts. “Injecting the MMR vaccine into the largest wart every 2 weeks has been found to lead to complete clearance in 60%-68% of cases in one study,” she said. “The benefit is that it’s $21 per dose, which is nice, but as with any vaccination, patients can develop flu-like symptoms as side effects.”
Use of the HPV vaccine for treating cutaneous warts remains controversial, she continued, but it seems to work better in younger patients. In one open-label study that evaluated the HPV vaccine for the treatment of multiple recalcitrant warts, with doses administered at 0. 2, and 6 months, the response rate 3 months after the third dose was 55% among those older than age 26, compared with 84% among those ages 9-26 years.
Another option, intralesional cidofovir, has been shown to be especially effective for refractory warts. “It has also been shown to work for warts in immunocompetent and immunocompromised patients,” Dr. Adams said.
In the realm of adjuvant treatments, microneedling has been found to have similar efficacy to needling, Dr. Adams said, but with minimal pain. “When we combine it with topical treatments like 5-FU, it’s even more efficacious,” she said.
One study found that combining microneedling with topical 5-FU had clearance similar to that of intralesional 5-FU or microneedling alone, but involved fewer treatment sessions and less pain in the combination group.
Autoinoculation has been used to stimulate an immune response in patients with warts, leading to clearance rates of 4% (mild clearance) to 66% (complete clearance) in one study. “We would expect this to work better in immunocompetent patients, but it’s something to keep in mind if you’re limited in the medications you can get for a patient,” Dr. Adams said. Also, results from a systematic review and meta-analysis suggest that systemic retinoids combined with intralesional immunotherapy leads to higher clearance rates and lower rates of recurrence of warts. The top performer among those tested was acitretin plus Candida antigen.
Dr. Adams advised dermatologists who try alternatives to salicylic acid and cryotherapy for warts to be “wary of a lack of high-level evidence” for their use. “They can be helpful for patients who have failed traditional therapies or have a contraindication to the usual go-tos.”
She reported having no relevant financial disclosures.
FROM AAD 2024
Time Is Money: Should Physicians Be Compensated for EHR Engagement?
Electronic health records (EHRs) make providing coordinated, efficient care easier and reduce medical errors and test duplications; research has also correlated EHR adoption with higher patient satisfaction and outcomes. However, for physicians, the benefits come at a cost.
Physicians spend significantly more time in healthcare portals, making notes, entering orders, reviewing clinical reports, and responding to patient messages.
“I spend at least the same amount of time in the portal that I do in scheduled clinical time with patients,” said Eve Rittenberg, MD, primary care physician at Brigham and Women’s Hospital and assistant professor at Harvard Medical School, Boston. “So, if I have a 4-hour session of seeing patients, I spend at least another 4 or more hours in the patient portal.”
The latest data showed that primary care physicians logged a median of 36.2 minutes in the healthcare portal per patient visit, spending 58.9% more time on orders, 24.4% more time reading and responding to messages, and 13% more time on chart review compared with prepandemic portal use.
“EHRs can be very powerful tools,” said Ralph DeBiasi, MD, a clinical cardiac electrophysiologist at Yale New Haven Health in Connecticut. “We’re still working on how to best harness that power to make us better doctors and better care teams and to take better care of our patients because their use can take up a lot of time.”
Portal Time Isn’t Paid Time
Sharp increases in the amount of time spent in the EHR responding to messages or dispensing medical advice via the portal often aren’t linked to increases in compensation; most portal time is unpaid.
“There isn’t specific time allocated to working in the portal; it’s either done in the office while a patient is sitting in an exam room or in the mornings and evenings outside of traditional working hours,” Dr. DeBiasi told this news organization. “I think it’s reasonable to consider it being reimbursed because we’re taking our time and effort and making decisions to help the patient.”
Compensation for portal time affects all physicians, but the degree of impact depends on their specialties. Primary care physicians spent significantly more daily and after-hours time in the EHR, entering notes and orders, and doing clinical reviews compared to surgical and medical specialties.
In addition to the outsized impact on primary care, physician compensation for portal time is also an equity issue.
Dr. Rittenberg researched the issue and found a higher volume of communication from both patients and staff to female physicians than male physicians. As a result, female physicians spend 41.4 minutes more on the EHR than their male counterparts, which equates to more unpaid time. It’s likely no coincidence then that burnout rates are also higher among female physicians, who also leave the clinical workforce in higher numbers, especially in primary care.
“Finding ways to fairly compensate physicians for their work also will address some of the equity issues in workload and the consequences,” Dr. Rittenberg said.
Addressing the Issue
Some health systems have started charging patients who seek medical advice via patient portals, equating the communication to asynchronous acute care or an additional care touch point and billing based on the length and complexity of the messages. Patient fees for seeking medical advice via portals vary widely depending on their health system and insurance.
At University of California San Francisco Health, billing patients for EHR communication led to a sharp decrease in patient messages, which eased physician workload. At Cleveland Clinic, physicians receive “productivity credits” for the time spent in the EHR that can be used to reduce their clinic hours (but have no impact on their compensation).
Changes to the Medicare Physician Fee Schedule also allow physicians to bill for “digital evaluation and management” based on the time spent in an EHR responding to patient-initiated questions and requests.
However, more efforts are needed to ease burnout and reverse the number of physicians who are seeing fewer patients or leaving medical practice altogether as a direct result of spending increasing amounts of unpaid time in the EHR. Dr. Rittenberg, who spends an estimated 50% of her working hours in the portal, had to reduce her clinical workload by 25% due to such heavy portal requirements.
“The workload has become unsustainable,” she said. “The work has undergone a dramatic change over the past decade, and the compensation system has not kept up with that change.”
Prioritizing Patient and Physician Experiences
The ever-expanding use of EHRs is a result of their value as a healthcare tool. Data showed that the electronic exchange of information between patients and physicians improves diagnostics, reduces medical errors, enhances communication, and leads to more patient-centered care — and physicians want their patients to use the portal to maximize their healthcare.
“[The EHR] is good for patients,” said Dr. DeBiasi. “Sometimes, patients have access issues with healthcare, whether that’s not knowing what number to call or getting the right message to the right person at the right office. If [the portal] is good for them and helps them get access to care, we should embrace that and figure out a way to work it into our day-to-day schedules.”
But maximizing the patient experience shouldn’t come at the physicians’ expense. Dr. Rittenberg advocates a model that compensates physicians for the time spent in the EHR and prioritizes a team approach to rebalance the EHR workload to ensure that physicians aren’t devoting too much time to administrative tasks and can, instead, focus their time on clinical tasks.
“The way in which we provide healthcare has fundamentally shifted, and compensation models need to reflect that new reality,” Dr. Rittenberg added.
A version of this article first appeared on Medscape.com.
Electronic health records (EHRs) make providing coordinated, efficient care easier and reduce medical errors and test duplications; research has also correlated EHR adoption with higher patient satisfaction and outcomes. However, for physicians, the benefits come at a cost.
Physicians spend significantly more time in healthcare portals, making notes, entering orders, reviewing clinical reports, and responding to patient messages.
“I spend at least the same amount of time in the portal that I do in scheduled clinical time with patients,” said Eve Rittenberg, MD, primary care physician at Brigham and Women’s Hospital and assistant professor at Harvard Medical School, Boston. “So, if I have a 4-hour session of seeing patients, I spend at least another 4 or more hours in the patient portal.”
The latest data showed that primary care physicians logged a median of 36.2 minutes in the healthcare portal per patient visit, spending 58.9% more time on orders, 24.4% more time reading and responding to messages, and 13% more time on chart review compared with prepandemic portal use.
“EHRs can be very powerful tools,” said Ralph DeBiasi, MD, a clinical cardiac electrophysiologist at Yale New Haven Health in Connecticut. “We’re still working on how to best harness that power to make us better doctors and better care teams and to take better care of our patients because their use can take up a lot of time.”
Portal Time Isn’t Paid Time
Sharp increases in the amount of time spent in the EHR responding to messages or dispensing medical advice via the portal often aren’t linked to increases in compensation; most portal time is unpaid.
“There isn’t specific time allocated to working in the portal; it’s either done in the office while a patient is sitting in an exam room or in the mornings and evenings outside of traditional working hours,” Dr. DeBiasi told this news organization. “I think it’s reasonable to consider it being reimbursed because we’re taking our time and effort and making decisions to help the patient.”
Compensation for portal time affects all physicians, but the degree of impact depends on their specialties. Primary care physicians spent significantly more daily and after-hours time in the EHR, entering notes and orders, and doing clinical reviews compared to surgical and medical specialties.
In addition to the outsized impact on primary care, physician compensation for portal time is also an equity issue.
Dr. Rittenberg researched the issue and found a higher volume of communication from both patients and staff to female physicians than male physicians. As a result, female physicians spend 41.4 minutes more on the EHR than their male counterparts, which equates to more unpaid time. It’s likely no coincidence then that burnout rates are also higher among female physicians, who also leave the clinical workforce in higher numbers, especially in primary care.
“Finding ways to fairly compensate physicians for their work also will address some of the equity issues in workload and the consequences,” Dr. Rittenberg said.
Addressing the Issue
Some health systems have started charging patients who seek medical advice via patient portals, equating the communication to asynchronous acute care or an additional care touch point and billing based on the length and complexity of the messages. Patient fees for seeking medical advice via portals vary widely depending on their health system and insurance.
At University of California San Francisco Health, billing patients for EHR communication led to a sharp decrease in patient messages, which eased physician workload. At Cleveland Clinic, physicians receive “productivity credits” for the time spent in the EHR that can be used to reduce their clinic hours (but have no impact on their compensation).
Changes to the Medicare Physician Fee Schedule also allow physicians to bill for “digital evaluation and management” based on the time spent in an EHR responding to patient-initiated questions and requests.
However, more efforts are needed to ease burnout and reverse the number of physicians who are seeing fewer patients or leaving medical practice altogether as a direct result of spending increasing amounts of unpaid time in the EHR. Dr. Rittenberg, who spends an estimated 50% of her working hours in the portal, had to reduce her clinical workload by 25% due to such heavy portal requirements.
“The workload has become unsustainable,” she said. “The work has undergone a dramatic change over the past decade, and the compensation system has not kept up with that change.”
Prioritizing Patient and Physician Experiences
The ever-expanding use of EHRs is a result of their value as a healthcare tool. Data showed that the electronic exchange of information between patients and physicians improves diagnostics, reduces medical errors, enhances communication, and leads to more patient-centered care — and physicians want their patients to use the portal to maximize their healthcare.
“[The EHR] is good for patients,” said Dr. DeBiasi. “Sometimes, patients have access issues with healthcare, whether that’s not knowing what number to call or getting the right message to the right person at the right office. If [the portal] is good for them and helps them get access to care, we should embrace that and figure out a way to work it into our day-to-day schedules.”
But maximizing the patient experience shouldn’t come at the physicians’ expense. Dr. Rittenberg advocates a model that compensates physicians for the time spent in the EHR and prioritizes a team approach to rebalance the EHR workload to ensure that physicians aren’t devoting too much time to administrative tasks and can, instead, focus their time on clinical tasks.
“The way in which we provide healthcare has fundamentally shifted, and compensation models need to reflect that new reality,” Dr. Rittenberg added.
A version of this article first appeared on Medscape.com.
Electronic health records (EHRs) make providing coordinated, efficient care easier and reduce medical errors and test duplications; research has also correlated EHR adoption with higher patient satisfaction and outcomes. However, for physicians, the benefits come at a cost.
Physicians spend significantly more time in healthcare portals, making notes, entering orders, reviewing clinical reports, and responding to patient messages.
“I spend at least the same amount of time in the portal that I do in scheduled clinical time with patients,” said Eve Rittenberg, MD, primary care physician at Brigham and Women’s Hospital and assistant professor at Harvard Medical School, Boston. “So, if I have a 4-hour session of seeing patients, I spend at least another 4 or more hours in the patient portal.”
The latest data showed that primary care physicians logged a median of 36.2 minutes in the healthcare portal per patient visit, spending 58.9% more time on orders, 24.4% more time reading and responding to messages, and 13% more time on chart review compared with prepandemic portal use.
“EHRs can be very powerful tools,” said Ralph DeBiasi, MD, a clinical cardiac electrophysiologist at Yale New Haven Health in Connecticut. “We’re still working on how to best harness that power to make us better doctors and better care teams and to take better care of our patients because their use can take up a lot of time.”
Portal Time Isn’t Paid Time
Sharp increases in the amount of time spent in the EHR responding to messages or dispensing medical advice via the portal often aren’t linked to increases in compensation; most portal time is unpaid.
“There isn’t specific time allocated to working in the portal; it’s either done in the office while a patient is sitting in an exam room or in the mornings and evenings outside of traditional working hours,” Dr. DeBiasi told this news organization. “I think it’s reasonable to consider it being reimbursed because we’re taking our time and effort and making decisions to help the patient.”
Compensation for portal time affects all physicians, but the degree of impact depends on their specialties. Primary care physicians spent significantly more daily and after-hours time in the EHR, entering notes and orders, and doing clinical reviews compared to surgical and medical specialties.
In addition to the outsized impact on primary care, physician compensation for portal time is also an equity issue.
Dr. Rittenberg researched the issue and found a higher volume of communication from both patients and staff to female physicians than male physicians. As a result, female physicians spend 41.4 minutes more on the EHR than their male counterparts, which equates to more unpaid time. It’s likely no coincidence then that burnout rates are also higher among female physicians, who also leave the clinical workforce in higher numbers, especially in primary care.
“Finding ways to fairly compensate physicians for their work also will address some of the equity issues in workload and the consequences,” Dr. Rittenberg said.
Addressing the Issue
Some health systems have started charging patients who seek medical advice via patient portals, equating the communication to asynchronous acute care or an additional care touch point and billing based on the length and complexity of the messages. Patient fees for seeking medical advice via portals vary widely depending on their health system and insurance.
At University of California San Francisco Health, billing patients for EHR communication led to a sharp decrease in patient messages, which eased physician workload. At Cleveland Clinic, physicians receive “productivity credits” for the time spent in the EHR that can be used to reduce their clinic hours (but have no impact on their compensation).
Changes to the Medicare Physician Fee Schedule also allow physicians to bill for “digital evaluation and management” based on the time spent in an EHR responding to patient-initiated questions and requests.
However, more efforts are needed to ease burnout and reverse the number of physicians who are seeing fewer patients or leaving medical practice altogether as a direct result of spending increasing amounts of unpaid time in the EHR. Dr. Rittenberg, who spends an estimated 50% of her working hours in the portal, had to reduce her clinical workload by 25% due to such heavy portal requirements.
“The workload has become unsustainable,” she said. “The work has undergone a dramatic change over the past decade, and the compensation system has not kept up with that change.”
Prioritizing Patient and Physician Experiences
The ever-expanding use of EHRs is a result of their value as a healthcare tool. Data showed that the electronic exchange of information between patients and physicians improves diagnostics, reduces medical errors, enhances communication, and leads to more patient-centered care — and physicians want their patients to use the portal to maximize their healthcare.
“[The EHR] is good for patients,” said Dr. DeBiasi. “Sometimes, patients have access issues with healthcare, whether that’s not knowing what number to call or getting the right message to the right person at the right office. If [the portal] is good for them and helps them get access to care, we should embrace that and figure out a way to work it into our day-to-day schedules.”
But maximizing the patient experience shouldn’t come at the physicians’ expense. Dr. Rittenberg advocates a model that compensates physicians for the time spent in the EHR and prioritizes a team approach to rebalance the EHR workload to ensure that physicians aren’t devoting too much time to administrative tasks and can, instead, focus their time on clinical tasks.
“The way in which we provide healthcare has fundamentally shifted, and compensation models need to reflect that new reality,” Dr. Rittenberg added.
A version of this article first appeared on Medscape.com.
Erenumab Linked to Better Migraine Prevention
TOPLINE:
Earlier treatment with erenumab was associated with significantly better migraine prevention than that with nonspecific oral migraine preventive medications (OMPMs) in patients with resistant episodic migraine. Based on this research, the investigators suggest clinicians should start erenumab early and not prolong use of OMPMs.
METHODOLOGY:
- The 12-month prospective, international, multicenter, phase 4 randomized clinical APPRAISE trial included 621 adult patients (mean age, 41 years; 88% female) with a ≥ 12-month history of migraine and between 4 and 15 monthly migraine days (MMDs).
- Primary endpoint was the proportion of patients who completed 12 months of the initially assigned treatment and experiencing a reduction of ≥ 50% from baseline in MMDs at the end of the year.
- Secondary endpoints included cumulative mean change from baseline in MMDs during the treatment period and the proportion of responders (based on the Patients’ Global Impression of Change scale) at month 12 for patients taking the initially assigned treatment.
TAKEAWAY:
- At month 12, patients receiving erenumab were six times more likely to report a ≥ 50% reduction in MMDs than those receiving OMPMs (odds ratio [OR], 6.48; P < .001).
- Compared with OMPMs, treatment with erenumab yielded a higher responder rate at 1 year (76% vs 19%; OR, 13.75; P < .001) and a significantly greater reduction in cumulative average MMDs (−4.32 days vs −2.65 days; P < .001).
- Substantially, fewer patients in the erenumab vs the OMPM group switched medication (2% vs 35%) or discontinued treatment due to adverse events (3% vs 23%).
- Incidence of treatment-emergent adverse events was similar between the treatment arms (75% vs 76%) until the researchers adjusted for exposure to treatment, which revealed a roughly 30% lower exposure-adjusted rate (per 100 patient-years) in the erenumab group.
IN PRACTICE:
“Earlier initiation of erenumab may ultimately lead to fewer patients discontinuing or switching medication in a real-world clinical practice,” the authors wrote. In addition, the findings “lend further support to the recent guideline update issued by the European Headache Federation, in which CGRP-targeted mAbs are considered a first-line treatment option for patients with migraine who require preventive treatment.”
SOURCE:
Patricia Pozo-Rosich, MD, PhD, of the Headache and Neurological Pain Research Group, Vall d’Hebron Institute of Research, Department of Medicine, Universitat Autònoma de Barcelona, Spain, and the Headache Unit, Neurology Department, Vall d’Hebron University Hospital, Barcelona, Spain, was the lead and corresponding author of the study. It was published online in JAMA Neurology.
LIMITATIONS:
Only locally approved and marketed OMPMs at study onset were used as comparators. The open-label study design might have led to a placebo response, which could have played a role in the findings because erenumab can only be administered in a clinic and was administered subcutaneously.
DISCLOSURES:
This study was funded by Novartis Pharma AG, Basel, Switzerland. Dr. Pozo-Rosich reported receiving grants from AbbVie, Novartis, and Teva and personal fees from AbbVie, Eli Lilly, Lundbeck, Novartis, Pfizer, and Teva outside the submitted work. The other authors’ disclosures were listed on the original paper.
A version of this article first appeared on Medscape.com.
TOPLINE:
Earlier treatment with erenumab was associated with significantly better migraine prevention than that with nonspecific oral migraine preventive medications (OMPMs) in patients with resistant episodic migraine. Based on this research, the investigators suggest clinicians should start erenumab early and not prolong use of OMPMs.
METHODOLOGY:
- The 12-month prospective, international, multicenter, phase 4 randomized clinical APPRAISE trial included 621 adult patients (mean age, 41 years; 88% female) with a ≥ 12-month history of migraine and between 4 and 15 monthly migraine days (MMDs).
- Primary endpoint was the proportion of patients who completed 12 months of the initially assigned treatment and experiencing a reduction of ≥ 50% from baseline in MMDs at the end of the year.
- Secondary endpoints included cumulative mean change from baseline in MMDs during the treatment period and the proportion of responders (based on the Patients’ Global Impression of Change scale) at month 12 for patients taking the initially assigned treatment.
TAKEAWAY:
- At month 12, patients receiving erenumab were six times more likely to report a ≥ 50% reduction in MMDs than those receiving OMPMs (odds ratio [OR], 6.48; P < .001).
- Compared with OMPMs, treatment with erenumab yielded a higher responder rate at 1 year (76% vs 19%; OR, 13.75; P < .001) and a significantly greater reduction in cumulative average MMDs (−4.32 days vs −2.65 days; P < .001).
- Substantially, fewer patients in the erenumab vs the OMPM group switched medication (2% vs 35%) or discontinued treatment due to adverse events (3% vs 23%).
- Incidence of treatment-emergent adverse events was similar between the treatment arms (75% vs 76%) until the researchers adjusted for exposure to treatment, which revealed a roughly 30% lower exposure-adjusted rate (per 100 patient-years) in the erenumab group.
IN PRACTICE:
“Earlier initiation of erenumab may ultimately lead to fewer patients discontinuing or switching medication in a real-world clinical practice,” the authors wrote. In addition, the findings “lend further support to the recent guideline update issued by the European Headache Federation, in which CGRP-targeted mAbs are considered a first-line treatment option for patients with migraine who require preventive treatment.”
SOURCE:
Patricia Pozo-Rosich, MD, PhD, of the Headache and Neurological Pain Research Group, Vall d’Hebron Institute of Research, Department of Medicine, Universitat Autònoma de Barcelona, Spain, and the Headache Unit, Neurology Department, Vall d’Hebron University Hospital, Barcelona, Spain, was the lead and corresponding author of the study. It was published online in JAMA Neurology.
LIMITATIONS:
Only locally approved and marketed OMPMs at study onset were used as comparators. The open-label study design might have led to a placebo response, which could have played a role in the findings because erenumab can only be administered in a clinic and was administered subcutaneously.
DISCLOSURES:
This study was funded by Novartis Pharma AG, Basel, Switzerland. Dr. Pozo-Rosich reported receiving grants from AbbVie, Novartis, and Teva and personal fees from AbbVie, Eli Lilly, Lundbeck, Novartis, Pfizer, and Teva outside the submitted work. The other authors’ disclosures were listed on the original paper.
A version of this article first appeared on Medscape.com.
TOPLINE:
Earlier treatment with erenumab was associated with significantly better migraine prevention than that with nonspecific oral migraine preventive medications (OMPMs) in patients with resistant episodic migraine. Based on this research, the investigators suggest clinicians should start erenumab early and not prolong use of OMPMs.
METHODOLOGY:
- The 12-month prospective, international, multicenter, phase 4 randomized clinical APPRAISE trial included 621 adult patients (mean age, 41 years; 88% female) with a ≥ 12-month history of migraine and between 4 and 15 monthly migraine days (MMDs).
- Primary endpoint was the proportion of patients who completed 12 months of the initially assigned treatment and experiencing a reduction of ≥ 50% from baseline in MMDs at the end of the year.
- Secondary endpoints included cumulative mean change from baseline in MMDs during the treatment period and the proportion of responders (based on the Patients’ Global Impression of Change scale) at month 12 for patients taking the initially assigned treatment.
TAKEAWAY:
- At month 12, patients receiving erenumab were six times more likely to report a ≥ 50% reduction in MMDs than those receiving OMPMs (odds ratio [OR], 6.48; P < .001).
- Compared with OMPMs, treatment with erenumab yielded a higher responder rate at 1 year (76% vs 19%; OR, 13.75; P < .001) and a significantly greater reduction in cumulative average MMDs (−4.32 days vs −2.65 days; P < .001).
- Substantially, fewer patients in the erenumab vs the OMPM group switched medication (2% vs 35%) or discontinued treatment due to adverse events (3% vs 23%).
- Incidence of treatment-emergent adverse events was similar between the treatment arms (75% vs 76%) until the researchers adjusted for exposure to treatment, which revealed a roughly 30% lower exposure-adjusted rate (per 100 patient-years) in the erenumab group.
IN PRACTICE:
“Earlier initiation of erenumab may ultimately lead to fewer patients discontinuing or switching medication in a real-world clinical practice,” the authors wrote. In addition, the findings “lend further support to the recent guideline update issued by the European Headache Federation, in which CGRP-targeted mAbs are considered a first-line treatment option for patients with migraine who require preventive treatment.”
SOURCE:
Patricia Pozo-Rosich, MD, PhD, of the Headache and Neurological Pain Research Group, Vall d’Hebron Institute of Research, Department of Medicine, Universitat Autònoma de Barcelona, Spain, and the Headache Unit, Neurology Department, Vall d’Hebron University Hospital, Barcelona, Spain, was the lead and corresponding author of the study. It was published online in JAMA Neurology.
LIMITATIONS:
Only locally approved and marketed OMPMs at study onset were used as comparators. The open-label study design might have led to a placebo response, which could have played a role in the findings because erenumab can only be administered in a clinic and was administered subcutaneously.
DISCLOSURES:
This study was funded by Novartis Pharma AG, Basel, Switzerland. Dr. Pozo-Rosich reported receiving grants from AbbVie, Novartis, and Teva and personal fees from AbbVie, Eli Lilly, Lundbeck, Novartis, Pfizer, and Teva outside the submitted work. The other authors’ disclosures were listed on the original paper.
A version of this article first appeared on Medscape.com.
ADHD Meds Linked to Lower Suicide, Hospitalization Risk
TOPLINE:
Certain stimulants prescribed for attention-deficit/hyperactivity disorder (ADHD) are associated with a decreased risk for psychiatric and nonpsychiatric hospitalization and suicide, new data from a national cohort study showed.
METHODOLOGY:
- Investigators used various medical and administrative databases in Sweden to identify individuals aged 16-65 years who were diagnosed with ADHD between January 2006 and December 2021.
- Participants were followed for up to 15 years (mean duration, 7 years) from date of diagnosis until death, emigration, or end of data linkage in December 2021.
- Researchers wanted to explore the link between ADHD meds and psychiatric hospitalization, nonpsychiatric hospitalization, and suicidal behavior.
TAKEAWAY:
- The cohort included 221,700 individuals with ADHD (mean age, 25 years; 54% male), and 56% had a psychiatric comorbidity such as an anxiety or stress-related disorder (24%), and depression or bipolar disorder (20%).
- Investigators found significantly lower risk for psychiatric hospitalization for the several medications. These included amphetamine (adjusted hazard ratio [aHR], 0.74), lisdexamphetamine (aHR, 0.80), dexamphetamine (aHR, 0.88), methylphenidate (aHR, 0.93), and polytherapy (aHR, 0.85). All but atomoxetine was significant at the P < .001 level.
- ADHD medications associated with a significantly lower risk for nonpsychiatric hospitalization included amphetamine (aHR, 0.62), lisdexamphetamine (aHR, 0.64), polytherapy (aHR, 0.67), dexamphetamine (aHR, 0.72), methylphenidate (aHR, 0.80), and atomoxetine (aHR, 0.84). All but atomoxetine was significant at the P < .001 level.
- Use of dexamphetamine (aHR, 0.69; P < .001), lisdexamphetamine (aHR, 0.76; P = .43), polytherapy (aHR, 0.85; P = .02), and methylphenidate (aHR, 0.92; P = .007) were associated with a significantly lower risk for suicidal behavior.
IN PRACTICE:
“Although concerns have been raised about the potential of amphetamines and methylphenidate for increasing the risk of adverse psychiatric outcomes, such as psychosis and mania, our results show that overall, the net effect on psychiatric outcomes is positive,” study authors wrote.
SOURCE:
Heidi Taipale, PhD, of Karolinska Institutet, led the study, which was published online in JAMA Network Open.
LIMITATIONS:
Due to the use of nationwide registers, there was a lack of detailed clinical data, including type and severity of symptoms. There was also no data on nonpharmacologic treatments.
DISCLOSURES:
The study was funded by the AFA Insurance Agency. Dr. Taipale reported receiving personal fees from Gedeon Richter, Janssen, Lundbeck, and Otsuka and grants from Janssen and Eli Lilly outside of the submitted work. Other disclosures are noted in the original article.
A version of this article first appeared on Medscape.com.
TOPLINE:
Certain stimulants prescribed for attention-deficit/hyperactivity disorder (ADHD) are associated with a decreased risk for psychiatric and nonpsychiatric hospitalization and suicide, new data from a national cohort study showed.
METHODOLOGY:
- Investigators used various medical and administrative databases in Sweden to identify individuals aged 16-65 years who were diagnosed with ADHD between January 2006 and December 2021.
- Participants were followed for up to 15 years (mean duration, 7 years) from date of diagnosis until death, emigration, or end of data linkage in December 2021.
- Researchers wanted to explore the link between ADHD meds and psychiatric hospitalization, nonpsychiatric hospitalization, and suicidal behavior.
TAKEAWAY:
- The cohort included 221,700 individuals with ADHD (mean age, 25 years; 54% male), and 56% had a psychiatric comorbidity such as an anxiety or stress-related disorder (24%), and depression or bipolar disorder (20%).
- Investigators found significantly lower risk for psychiatric hospitalization for the several medications. These included amphetamine (adjusted hazard ratio [aHR], 0.74), lisdexamphetamine (aHR, 0.80), dexamphetamine (aHR, 0.88), methylphenidate (aHR, 0.93), and polytherapy (aHR, 0.85). All but atomoxetine was significant at the P < .001 level.
- ADHD medications associated with a significantly lower risk for nonpsychiatric hospitalization included amphetamine (aHR, 0.62), lisdexamphetamine (aHR, 0.64), polytherapy (aHR, 0.67), dexamphetamine (aHR, 0.72), methylphenidate (aHR, 0.80), and atomoxetine (aHR, 0.84). All but atomoxetine was significant at the P < .001 level.
- Use of dexamphetamine (aHR, 0.69; P < .001), lisdexamphetamine (aHR, 0.76; P = .43), polytherapy (aHR, 0.85; P = .02), and methylphenidate (aHR, 0.92; P = .007) were associated with a significantly lower risk for suicidal behavior.
IN PRACTICE:
“Although concerns have been raised about the potential of amphetamines and methylphenidate for increasing the risk of adverse psychiatric outcomes, such as psychosis and mania, our results show that overall, the net effect on psychiatric outcomes is positive,” study authors wrote.
SOURCE:
Heidi Taipale, PhD, of Karolinska Institutet, led the study, which was published online in JAMA Network Open.
LIMITATIONS:
Due to the use of nationwide registers, there was a lack of detailed clinical data, including type and severity of symptoms. There was also no data on nonpharmacologic treatments.
DISCLOSURES:
The study was funded by the AFA Insurance Agency. Dr. Taipale reported receiving personal fees from Gedeon Richter, Janssen, Lundbeck, and Otsuka and grants from Janssen and Eli Lilly outside of the submitted work. Other disclosures are noted in the original article.
A version of this article first appeared on Medscape.com.
TOPLINE:
Certain stimulants prescribed for attention-deficit/hyperactivity disorder (ADHD) are associated with a decreased risk for psychiatric and nonpsychiatric hospitalization and suicide, new data from a national cohort study showed.
METHODOLOGY:
- Investigators used various medical and administrative databases in Sweden to identify individuals aged 16-65 years who were diagnosed with ADHD between January 2006 and December 2021.
- Participants were followed for up to 15 years (mean duration, 7 years) from date of diagnosis until death, emigration, or end of data linkage in December 2021.
- Researchers wanted to explore the link between ADHD meds and psychiatric hospitalization, nonpsychiatric hospitalization, and suicidal behavior.
TAKEAWAY:
- The cohort included 221,700 individuals with ADHD (mean age, 25 years; 54% male), and 56% had a psychiatric comorbidity such as an anxiety or stress-related disorder (24%), and depression or bipolar disorder (20%).
- Investigators found significantly lower risk for psychiatric hospitalization for the several medications. These included amphetamine (adjusted hazard ratio [aHR], 0.74), lisdexamphetamine (aHR, 0.80), dexamphetamine (aHR, 0.88), methylphenidate (aHR, 0.93), and polytherapy (aHR, 0.85). All but atomoxetine was significant at the P < .001 level.
- ADHD medications associated with a significantly lower risk for nonpsychiatric hospitalization included amphetamine (aHR, 0.62), lisdexamphetamine (aHR, 0.64), polytherapy (aHR, 0.67), dexamphetamine (aHR, 0.72), methylphenidate (aHR, 0.80), and atomoxetine (aHR, 0.84). All but atomoxetine was significant at the P < .001 level.
- Use of dexamphetamine (aHR, 0.69; P < .001), lisdexamphetamine (aHR, 0.76; P = .43), polytherapy (aHR, 0.85; P = .02), and methylphenidate (aHR, 0.92; P = .007) were associated with a significantly lower risk for suicidal behavior.
IN PRACTICE:
“Although concerns have been raised about the potential of amphetamines and methylphenidate for increasing the risk of adverse psychiatric outcomes, such as psychosis and mania, our results show that overall, the net effect on psychiatric outcomes is positive,” study authors wrote.
SOURCE:
Heidi Taipale, PhD, of Karolinska Institutet, led the study, which was published online in JAMA Network Open.
LIMITATIONS:
Due to the use of nationwide registers, there was a lack of detailed clinical data, including type and severity of symptoms. There was also no data on nonpharmacologic treatments.
DISCLOSURES:
The study was funded by the AFA Insurance Agency. Dr. Taipale reported receiving personal fees from Gedeon Richter, Janssen, Lundbeck, and Otsuka and grants from Janssen and Eli Lilly outside of the submitted work. Other disclosures are noted in the original article.
A version of this article first appeared on Medscape.com.
The Nose Knows
A few weeks ago I stumbled upon a two-sentence blurb in a pediatric newsletter summarizing the results of a study comparing the chemical profile of infant body odor with that of postpubertal adolescents. The investigators found that, not surprisingly, the smell of the chemical constituents wafting from babies was more appealing than that emanating from sweaty teenagers. I quickly moved on to the next blurb hoping to find something I hadn’t already experienced or figured out on my own.
But, as I navigated through the rest of my day filled with pickleball, bicycling, and the smell of home-cooked food, something about that study of body odor nagged at me. Who had funded that voyage into the obvious? Were my tax dollars involved? Had I been duped by some alleged nonprofit that had promised my donation would save lives or at least ameliorate suffering? Finally, as the sun dipped below the horizon, my curiosity got the best of me and I searched out the original study. Within minutes I fell down a rabbit hole into the cavernous world of odor science.
Having had zero experience in this niche field, I was amazed at the lengths to which these German odor investigators had gone to analyze the chemicals on and around their subjects. Just trying to ensure that materials and microclimates in the experimental environment were scent-free was a heroic effort. There was “Mono-trap sampling of volatiles, followed by thermodesorption-comprehensive gas chromatography, and time of flight-mass spectrometry analysis.” There were graphs and charts galore. This is serious science, folks. However, they still use the abbreviation “BO” freely, just as I learned to do in junior high. And, in some situations, the investigators relied on the observation of a panel of trained human sniffers to assess the detection threshold and the degree of pleasantness.
Ultimately, the authors’ conclusion was “sexual maturation coincides with changes in body odor chemical composition. Whether those changes explain differences in parental olfactory perception needs to be determined in future studies.” Again, no surprises here.
Exhausted by my venture into the realm of odor science, I finally found the answer to my burning question. The study was supported by the German Research Foundation and the European Union. Phew! Not on my nickel.
Lest you think that I believe any investigation into the potential role of smell in our health and well-being is pure bunk, let me make it clear that I think the role of odor detection is one of the least well-studied and potentially most valuable areas of medical research. Having had one family tell me that their black lab had twice successfully “diagnosed” their pre-verbal child’s ear infection (which I confirmed with an otoscope and the tympanic membrane was intact) I have been keenly interested in the role of animal-assisted diagnosis.
If you also have wondered whether you could write off your pedigreed Portuguese Water Dog as an office expense, I would direct you to an article titled “Canine olfactory detection and its relevance to medical detection.” The authors note that there is some evidence of dogs successfully alerting physicians to Parkinson’s disease, some cancers, malaria, and COVID-19, among others. However, they caution that the reliability is, in most cases, not of a quality that would be helpful on a larger scale.
I can understand the reasons for their caution. However, from my own personal experience, I am completely confident that I can diagnose strep throat by smell, sometimes simply on opening the examination room door. My false-positive rate over 40 years of practice is zero. Of course I still test and, not surprisingly, my false-negative rate is nothing to brag about. However, if a dog can produce even close to my zero false negative with a given disease, that information is valuable and suggests that we should be pointing the odor investigators and their tool box of skills in that direction. I’m pretty sure we don’t need them to dig much deeper into why babies smell better than teenagers.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at pdnews@mdedge.com.
A few weeks ago I stumbled upon a two-sentence blurb in a pediatric newsletter summarizing the results of a study comparing the chemical profile of infant body odor with that of postpubertal adolescents. The investigators found that, not surprisingly, the smell of the chemical constituents wafting from babies was more appealing than that emanating from sweaty teenagers. I quickly moved on to the next blurb hoping to find something I hadn’t already experienced or figured out on my own.
But, as I navigated through the rest of my day filled with pickleball, bicycling, and the smell of home-cooked food, something about that study of body odor nagged at me. Who had funded that voyage into the obvious? Were my tax dollars involved? Had I been duped by some alleged nonprofit that had promised my donation would save lives or at least ameliorate suffering? Finally, as the sun dipped below the horizon, my curiosity got the best of me and I searched out the original study. Within minutes I fell down a rabbit hole into the cavernous world of odor science.
Having had zero experience in this niche field, I was amazed at the lengths to which these German odor investigators had gone to analyze the chemicals on and around their subjects. Just trying to ensure that materials and microclimates in the experimental environment were scent-free was a heroic effort. There was “Mono-trap sampling of volatiles, followed by thermodesorption-comprehensive gas chromatography, and time of flight-mass spectrometry analysis.” There were graphs and charts galore. This is serious science, folks. However, they still use the abbreviation “BO” freely, just as I learned to do in junior high. And, in some situations, the investigators relied on the observation of a panel of trained human sniffers to assess the detection threshold and the degree of pleasantness.
Ultimately, the authors’ conclusion was “sexual maturation coincides with changes in body odor chemical composition. Whether those changes explain differences in parental olfactory perception needs to be determined in future studies.” Again, no surprises here.
Exhausted by my venture into the realm of odor science, I finally found the answer to my burning question. The study was supported by the German Research Foundation and the European Union. Phew! Not on my nickel.
Lest you think that I believe any investigation into the potential role of smell in our health and well-being is pure bunk, let me make it clear that I think the role of odor detection is one of the least well-studied and potentially most valuable areas of medical research. Having had one family tell me that their black lab had twice successfully “diagnosed” their pre-verbal child’s ear infection (which I confirmed with an otoscope and the tympanic membrane was intact) I have been keenly interested in the role of animal-assisted diagnosis.
If you also have wondered whether you could write off your pedigreed Portuguese Water Dog as an office expense, I would direct you to an article titled “Canine olfactory detection and its relevance to medical detection.” The authors note that there is some evidence of dogs successfully alerting physicians to Parkinson’s disease, some cancers, malaria, and COVID-19, among others. However, they caution that the reliability is, in most cases, not of a quality that would be helpful on a larger scale.
I can understand the reasons for their caution. However, from my own personal experience, I am completely confident that I can diagnose strep throat by smell, sometimes simply on opening the examination room door. My false-positive rate over 40 years of practice is zero. Of course I still test and, not surprisingly, my false-negative rate is nothing to brag about. However, if a dog can produce even close to my zero false negative with a given disease, that information is valuable and suggests that we should be pointing the odor investigators and their tool box of skills in that direction. I’m pretty sure we don’t need them to dig much deeper into why babies smell better than teenagers.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at pdnews@mdedge.com.
A few weeks ago I stumbled upon a two-sentence blurb in a pediatric newsletter summarizing the results of a study comparing the chemical profile of infant body odor with that of postpubertal adolescents. The investigators found that, not surprisingly, the smell of the chemical constituents wafting from babies was more appealing than that emanating from sweaty teenagers. I quickly moved on to the next blurb hoping to find something I hadn’t already experienced or figured out on my own.
But, as I navigated through the rest of my day filled with pickleball, bicycling, and the smell of home-cooked food, something about that study of body odor nagged at me. Who had funded that voyage into the obvious? Were my tax dollars involved? Had I been duped by some alleged nonprofit that had promised my donation would save lives or at least ameliorate suffering? Finally, as the sun dipped below the horizon, my curiosity got the best of me and I searched out the original study. Within minutes I fell down a rabbit hole into the cavernous world of odor science.
Having had zero experience in this niche field, I was amazed at the lengths to which these German odor investigators had gone to analyze the chemicals on and around their subjects. Just trying to ensure that materials and microclimates in the experimental environment were scent-free was a heroic effort. There was “Mono-trap sampling of volatiles, followed by thermodesorption-comprehensive gas chromatography, and time of flight-mass spectrometry analysis.” There were graphs and charts galore. This is serious science, folks. However, they still use the abbreviation “BO” freely, just as I learned to do in junior high. And, in some situations, the investigators relied on the observation of a panel of trained human sniffers to assess the detection threshold and the degree of pleasantness.
Ultimately, the authors’ conclusion was “sexual maturation coincides with changes in body odor chemical composition. Whether those changes explain differences in parental olfactory perception needs to be determined in future studies.” Again, no surprises here.
Exhausted by my venture into the realm of odor science, I finally found the answer to my burning question. The study was supported by the German Research Foundation and the European Union. Phew! Not on my nickel.
Lest you think that I believe any investigation into the potential role of smell in our health and well-being is pure bunk, let me make it clear that I think the role of odor detection is one of the least well-studied and potentially most valuable areas of medical research. Having had one family tell me that their black lab had twice successfully “diagnosed” their pre-verbal child’s ear infection (which I confirmed with an otoscope and the tympanic membrane was intact) I have been keenly interested in the role of animal-assisted diagnosis.
If you also have wondered whether you could write off your pedigreed Portuguese Water Dog as an office expense, I would direct you to an article titled “Canine olfactory detection and its relevance to medical detection.” The authors note that there is some evidence of dogs successfully alerting physicians to Parkinson’s disease, some cancers, malaria, and COVID-19, among others. However, they caution that the reliability is, in most cases, not of a quality that would be helpful on a larger scale.
I can understand the reasons for their caution. However, from my own personal experience, I am completely confident that I can diagnose strep throat by smell, sometimes simply on opening the examination room door. My false-positive rate over 40 years of practice is zero. Of course I still test and, not surprisingly, my false-negative rate is nothing to brag about. However, if a dog can produce even close to my zero false negative with a given disease, that information is valuable and suggests that we should be pointing the odor investigators and their tool box of skills in that direction. I’m pretty sure we don’t need them to dig much deeper into why babies smell better than teenagers.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at pdnews@mdedge.com.
Formulation of Sclerotherapy Agent Found Useful for Submental Fat Reduction
SAN DIEGO — A .
The solution, also known as 10XB101, “has been demonstrated to cause adipolysis,” Kavita Darji, MD, an American Society for Dermatologic Surgery (ASDS) cosmetic and dermatologic laser surgery fellow at a practice in San Diego, said during a late-breaking session at the annual meeting of the American Academy of Dermatology. “It shows less inflammation and release of cytokines TNF-alpha and MCP-1 by macrophages than deoxycholic acid, which is currently used for submental fat reduction.”
In a phase 2b clinical trial conducted at four sites, investigators enrolled 51 patients and assigned them to one of four dose cohorts: 2%, 3%, or 4.5% 10XB101, or vehicle. Each treatment consisted of up to 50 injections at 0.2 mL per injection, and they were administered up to six times 4 weeks apart. Study endpoints included a composite of the Clinician Submental Fat Score (CSFS) and Patient Submental Fat Score (PSFS) on a 0-4–point scale. The researchers graded local skin reactions such as erythema, edema, tenderness on palpation, bruising, pain, and burning/stinging as 0 (none), 1 (mild), 2 (moderate), or 3 (severe). They also obtained lab tests and performed electrocardiograms.
Dr. Darji and colleagues analyzed two populations: the intent to treat (ITT) population, which included all 51 enrolled subjects who received any injection of the test agent, and a completer population of 40 subjects. “These patients had at least four treatments or completed the treatments per protocol, completed the 4 weeks after final treatment assessments, or did not have any significant protocol deviations that would impact the evaluation of efficacy,” she explained.
To compare how 10XB101 performed compared with deoxycholic acid (Kybella), which is approved by the FDA to improve the appearance of moderate to severe submental fat, the researchers drew from pooled findings of Refine 1 and 2, in which adults received up to six treatment sessions with deoxycholic acid or placebo.
The ITT analysis of the 3% and 4.5% 10XB101 dose groups showed about a fourfold increase in a 2-grade or better improvement in the composite endpoint relative to the pooled findings of deoxycholic acid (62% vs. 16%, respectively). In addition, 80% of the completer population achieved a 2-grade improvement in the composite endpoint. “Importantly, 10% to 33% also received a 3-grade improvement, depending on the dose they were assigned to,” Dr. Darji said.
On average, patients in both cohorts achieved a 1-grade improvement after two treatments, and about 50% achieved a 2-grade improvement after four treatments — which is consistent with a more rapid onset when compared with deoxycholic acid, she said.
Both study endpoints were achieved by 31% of patients in the ITT group vs. 33% of completers, respectively, with the 2% dose; 62% vs. 80% with the 3% dose; and 54% vs. 79% for the 4.5% dose. “This is a 2- to 5-times increase in success” when compared with the results of deoxycholic acid in the published pooled analysis, Dr. Darji said. The researchers measured adverse events by spontaneous and elicited reports and by assessments of recorded local skin reactions. They found that 80% of all measured local skin reactions rated as 0 while 98% of all measured local skin reactions rated as a 0 or 1. One myocardial infarction occurred, which was mild and resolved. This case was not related to the study drug, Dr. Darji said in an interview after the meeting. Otherwise, no safety laboratory or ECG signals were noted.
In findings limited to the 3% dose of 10XB101, mild bruising occurred in 8% of patients at postinjection visit 2, 18% of those at postinjection visit 3, 20% of those at postinjection visit 4, and in 20% of those at postinjection visit 5. Reports of mild pain/burning/stinging occurred in 8% of patients at postinjection visit 2 but at no other subsequent visits. Meanwhile, edema occurred in 42% of patients at postinjection visit 2, 45% of those at postinjection visit 3, 50% of those at postinjection visits 4 and 5, and 38% of those at postinjection visit 6.
“Patients resumed normal activity within 1-3 days and had fewer side effects that lasted longer than 30 days,” Dr. Darji concluded, adding that the results “imply a potential opportunity to expand the treatment to other anatomic areas, which is a future direction.”
One of the session moderators, Andrew Blauvelt, MD, MBA, of Oregon Medical Research Center, Portland, noted that the study was not a head-to-head trial of polidocanol vs. deoxycholic acid, so he cautioned against drawing strong conclusions about the comparative data presented.
Asked to comment on the results Lawrence J. Green, MD, of the department of dermatology at George Washington University, Washington, said that 10XB101 “showed excellent efficacy with much fewer adverse events when compared to what we found in studies with Kybella, the only currently FDA-approved injection for submental fat reduction.”
In addition, “much less pain after injection was to me the most obvious differentiator between this and Kybella studies. I look forward to seeing if larger studies confirm the efficacy and safety from this phase 2 study, as 10XB101 has potential to be a more effective, and safer option to reduce submental fat,” he added. He was not involved with the study.
Dr. Darji reported having no disclosures. Mitchel P. Goldman, MD, the study’s lead investigator, is a minority investor in 10XBio, which is developing 10XB101. Dr. Blauvelt and Dr. Green disclosed conflicts of interest from many pharmaceutical companies.
SAN DIEGO — A .
The solution, also known as 10XB101, “has been demonstrated to cause adipolysis,” Kavita Darji, MD, an American Society for Dermatologic Surgery (ASDS) cosmetic and dermatologic laser surgery fellow at a practice in San Diego, said during a late-breaking session at the annual meeting of the American Academy of Dermatology. “It shows less inflammation and release of cytokines TNF-alpha and MCP-1 by macrophages than deoxycholic acid, which is currently used for submental fat reduction.”
In a phase 2b clinical trial conducted at four sites, investigators enrolled 51 patients and assigned them to one of four dose cohorts: 2%, 3%, or 4.5% 10XB101, or vehicle. Each treatment consisted of up to 50 injections at 0.2 mL per injection, and they were administered up to six times 4 weeks apart. Study endpoints included a composite of the Clinician Submental Fat Score (CSFS) and Patient Submental Fat Score (PSFS) on a 0-4–point scale. The researchers graded local skin reactions such as erythema, edema, tenderness on palpation, bruising, pain, and burning/stinging as 0 (none), 1 (mild), 2 (moderate), or 3 (severe). They also obtained lab tests and performed electrocardiograms.
Dr. Darji and colleagues analyzed two populations: the intent to treat (ITT) population, which included all 51 enrolled subjects who received any injection of the test agent, and a completer population of 40 subjects. “These patients had at least four treatments or completed the treatments per protocol, completed the 4 weeks after final treatment assessments, or did not have any significant protocol deviations that would impact the evaluation of efficacy,” she explained.
To compare how 10XB101 performed compared with deoxycholic acid (Kybella), which is approved by the FDA to improve the appearance of moderate to severe submental fat, the researchers drew from pooled findings of Refine 1 and 2, in which adults received up to six treatment sessions with deoxycholic acid or placebo.
The ITT analysis of the 3% and 4.5% 10XB101 dose groups showed about a fourfold increase in a 2-grade or better improvement in the composite endpoint relative to the pooled findings of deoxycholic acid (62% vs. 16%, respectively). In addition, 80% of the completer population achieved a 2-grade improvement in the composite endpoint. “Importantly, 10% to 33% also received a 3-grade improvement, depending on the dose they were assigned to,” Dr. Darji said.
On average, patients in both cohorts achieved a 1-grade improvement after two treatments, and about 50% achieved a 2-grade improvement after four treatments — which is consistent with a more rapid onset when compared with deoxycholic acid, she said.
Both study endpoints were achieved by 31% of patients in the ITT group vs. 33% of completers, respectively, with the 2% dose; 62% vs. 80% with the 3% dose; and 54% vs. 79% for the 4.5% dose. “This is a 2- to 5-times increase in success” when compared with the results of deoxycholic acid in the published pooled analysis, Dr. Darji said. The researchers measured adverse events by spontaneous and elicited reports and by assessments of recorded local skin reactions. They found that 80% of all measured local skin reactions rated as 0 while 98% of all measured local skin reactions rated as a 0 or 1. One myocardial infarction occurred, which was mild and resolved. This case was not related to the study drug, Dr. Darji said in an interview after the meeting. Otherwise, no safety laboratory or ECG signals were noted.
In findings limited to the 3% dose of 10XB101, mild bruising occurred in 8% of patients at postinjection visit 2, 18% of those at postinjection visit 3, 20% of those at postinjection visit 4, and in 20% of those at postinjection visit 5. Reports of mild pain/burning/stinging occurred in 8% of patients at postinjection visit 2 but at no other subsequent visits. Meanwhile, edema occurred in 42% of patients at postinjection visit 2, 45% of those at postinjection visit 3, 50% of those at postinjection visits 4 and 5, and 38% of those at postinjection visit 6.
“Patients resumed normal activity within 1-3 days and had fewer side effects that lasted longer than 30 days,” Dr. Darji concluded, adding that the results “imply a potential opportunity to expand the treatment to other anatomic areas, which is a future direction.”
One of the session moderators, Andrew Blauvelt, MD, MBA, of Oregon Medical Research Center, Portland, noted that the study was not a head-to-head trial of polidocanol vs. deoxycholic acid, so he cautioned against drawing strong conclusions about the comparative data presented.
Asked to comment on the results Lawrence J. Green, MD, of the department of dermatology at George Washington University, Washington, said that 10XB101 “showed excellent efficacy with much fewer adverse events when compared to what we found in studies with Kybella, the only currently FDA-approved injection for submental fat reduction.”
In addition, “much less pain after injection was to me the most obvious differentiator between this and Kybella studies. I look forward to seeing if larger studies confirm the efficacy and safety from this phase 2 study, as 10XB101 has potential to be a more effective, and safer option to reduce submental fat,” he added. He was not involved with the study.
Dr. Darji reported having no disclosures. Mitchel P. Goldman, MD, the study’s lead investigator, is a minority investor in 10XBio, which is developing 10XB101. Dr. Blauvelt and Dr. Green disclosed conflicts of interest from many pharmaceutical companies.
SAN DIEGO — A .
The solution, also known as 10XB101, “has been demonstrated to cause adipolysis,” Kavita Darji, MD, an American Society for Dermatologic Surgery (ASDS) cosmetic and dermatologic laser surgery fellow at a practice in San Diego, said during a late-breaking session at the annual meeting of the American Academy of Dermatology. “It shows less inflammation and release of cytokines TNF-alpha and MCP-1 by macrophages than deoxycholic acid, which is currently used for submental fat reduction.”
In a phase 2b clinical trial conducted at four sites, investigators enrolled 51 patients and assigned them to one of four dose cohorts: 2%, 3%, or 4.5% 10XB101, or vehicle. Each treatment consisted of up to 50 injections at 0.2 mL per injection, and they were administered up to six times 4 weeks apart. Study endpoints included a composite of the Clinician Submental Fat Score (CSFS) and Patient Submental Fat Score (PSFS) on a 0-4–point scale. The researchers graded local skin reactions such as erythema, edema, tenderness on palpation, bruising, pain, and burning/stinging as 0 (none), 1 (mild), 2 (moderate), or 3 (severe). They also obtained lab tests and performed electrocardiograms.
Dr. Darji and colleagues analyzed two populations: the intent to treat (ITT) population, which included all 51 enrolled subjects who received any injection of the test agent, and a completer population of 40 subjects. “These patients had at least four treatments or completed the treatments per protocol, completed the 4 weeks after final treatment assessments, or did not have any significant protocol deviations that would impact the evaluation of efficacy,” she explained.
To compare how 10XB101 performed compared with deoxycholic acid (Kybella), which is approved by the FDA to improve the appearance of moderate to severe submental fat, the researchers drew from pooled findings of Refine 1 and 2, in which adults received up to six treatment sessions with deoxycholic acid or placebo.
The ITT analysis of the 3% and 4.5% 10XB101 dose groups showed about a fourfold increase in a 2-grade or better improvement in the composite endpoint relative to the pooled findings of deoxycholic acid (62% vs. 16%, respectively). In addition, 80% of the completer population achieved a 2-grade improvement in the composite endpoint. “Importantly, 10% to 33% also received a 3-grade improvement, depending on the dose they were assigned to,” Dr. Darji said.
On average, patients in both cohorts achieved a 1-grade improvement after two treatments, and about 50% achieved a 2-grade improvement after four treatments — which is consistent with a more rapid onset when compared with deoxycholic acid, she said.
Both study endpoints were achieved by 31% of patients in the ITT group vs. 33% of completers, respectively, with the 2% dose; 62% vs. 80% with the 3% dose; and 54% vs. 79% for the 4.5% dose. “This is a 2- to 5-times increase in success” when compared with the results of deoxycholic acid in the published pooled analysis, Dr. Darji said. The researchers measured adverse events by spontaneous and elicited reports and by assessments of recorded local skin reactions. They found that 80% of all measured local skin reactions rated as 0 while 98% of all measured local skin reactions rated as a 0 or 1. One myocardial infarction occurred, which was mild and resolved. This case was not related to the study drug, Dr. Darji said in an interview after the meeting. Otherwise, no safety laboratory or ECG signals were noted.
In findings limited to the 3% dose of 10XB101, mild bruising occurred in 8% of patients at postinjection visit 2, 18% of those at postinjection visit 3, 20% of those at postinjection visit 4, and in 20% of those at postinjection visit 5. Reports of mild pain/burning/stinging occurred in 8% of patients at postinjection visit 2 but at no other subsequent visits. Meanwhile, edema occurred in 42% of patients at postinjection visit 2, 45% of those at postinjection visit 3, 50% of those at postinjection visits 4 and 5, and 38% of those at postinjection visit 6.
“Patients resumed normal activity within 1-3 days and had fewer side effects that lasted longer than 30 days,” Dr. Darji concluded, adding that the results “imply a potential opportunity to expand the treatment to other anatomic areas, which is a future direction.”
One of the session moderators, Andrew Blauvelt, MD, MBA, of Oregon Medical Research Center, Portland, noted that the study was not a head-to-head trial of polidocanol vs. deoxycholic acid, so he cautioned against drawing strong conclusions about the comparative data presented.
Asked to comment on the results Lawrence J. Green, MD, of the department of dermatology at George Washington University, Washington, said that 10XB101 “showed excellent efficacy with much fewer adverse events when compared to what we found in studies with Kybella, the only currently FDA-approved injection for submental fat reduction.”
In addition, “much less pain after injection was to me the most obvious differentiator between this and Kybella studies. I look forward to seeing if larger studies confirm the efficacy and safety from this phase 2 study, as 10XB101 has potential to be a more effective, and safer option to reduce submental fat,” he added. He was not involved with the study.
Dr. Darji reported having no disclosures. Mitchel P. Goldman, MD, the study’s lead investigator, is a minority investor in 10XBio, which is developing 10XB101. Dr. Blauvelt and Dr. Green disclosed conflicts of interest from many pharmaceutical companies.
FROM AAD 2024
Early Biologic Initiation Linked to Rapid Improvement of JIA, Sustained Remission
CORRECTED April 16, 2024 // An earlier version of this article stated incorrect percentages of patients who never received any biologics during the study's 3-year period but improved rapidly or moderately.
Early initiation of biologics — within the first 2 months of symptom presentation — appears to have a significant impact on how rapidly patients with juvenile idiopathic arthritis (JIA) improve, according to findings presented at the annual scientific meeting of the Childhood Arthritis and Rheumatology Research Alliance.
“Our study provides evidence that early use of biologics can significantly alter the disease trajectory of patients with JIA,” Mei-Sing Ong, PhD, of Harvard Medical School, Boston, told attendees. At the same time, however, not all patients who improved rapidly during a 3-year follow-up period needed biologics, a finding that Ong said the researchers are continuing to investigate.
Marinka Twilt, MD, MScE, PhD, chair of CARRA’s JIA Research Committee and a pediatric rheumatologist and clinician scientist at Alberta Children’s Hospital in Calgary, Canada, was not involved in the research but said the continued sustained remission in patients who improved rapidly is very reassuring.
“We always wonder if initial response will be sustained or if patients tend to flare after the initial treatment,” Dr. Twilt told this news organization. “To see the sustained response up to 3 years is fantastic.” She added that it would be enlightening to see more information about patients who rapidly improved over 3 years, including whether they were still taking a [conventional disease-modifying antirheumatic drug (DMARD)] and/or biologic.
“A new diagnosis can be overwhelming for families, and this sometimes leads to step-up therapy to not overwhelm them more with information on new drugs,” Dr. Twilt said. “This study shows that an earlier start is beneficial, and this should be discussed with families early on so there is less delay in early treatment.”
Canada and many US states currently require 3 months of conventional DMARD treatment before patients can start a biologic, Dr. Twilt said, yet “this study shows the additive benefit of using a biologic within 2 months of starting a DMARD, which hopefully will lead to insurance companies adopting this threshold.”
The STOP-JIA study is a prospective observational study that compares the effectiveness of three different treatment plans for JIA. A Step-Up cohort of 257 patients received conventional antirheumatic monotherapy initially, with a biologic added at 3 months or later as needed. The Early Combination cohort of 100 patients received conventional antirheumatic therapy with a biologic from the start. The Biologic First cohort of 43 patients began taking a biologic as a first-line therapy.
In previously reported results of the study at 12 months’ follow-up, there was no significant difference between the Step-Up and Biologic First groups, but there were significant differences between the Step-Up and Early Combination groups. Significantly more patients in the Early Combination group (58.8%) than in the Step-Up group (42.8%) had inactive disease, based on the clinical Juvenile Arthritis Disease Activity Score 10 (cJADAS-10) (P = .03). Similarly, 81% of Early Combination patients achieved the American College of Rheumatology 70% improvement criteria, compared with 62% of the Step-Up patients (P = .01).
To learn whether the timing of starting a biologic influenced the disease trajectory over time, the researchers compared subgroups of patients with similar trajectories.
“Assessing treatment outcomes at a single point in time does not give us a complete picture of the effects of treatment on disease trajectory, which is an important outcome given that JIA is characterized by a relapsing-remitting course,” Dr. Ong told attendees.
Patients were sorted in the slow, moderate, or rapid improvement trajectories. In previously reported data at 12 months’ follow-up, patients’ odds of achieving rapid improvement were 3.6 times greater if they had started a biologic within 3 months.
This study compared patients’ trajectories over 3 years in the 259 patients (65% of the original cohort) who had at least one cJADAS-10 assessment in each year of follow-up. Most patients (66.8%) were in the rapid improvement class, with 25.9% in the moderate improvement class and 7.3% in the slow improvement class.
Patients in the rapid improvement group achieved inactive disease (cJADAS-10 of 2.5 or less) within 1 year and maintained inactive disease through the second and third years. The moderate and low improvement groups both had higher disease activity at baseline, but the moderate group continued to improve in years 2 and 3, with minimal disease by year 3, on the basis of the cJADAS-10 scores of 2.5-5. The slow group continued to experience moderate disease activity during years 2 and 3.
The findings also revealed that the earlier patients began a biologic, the more likely they were to be in the rapid improvement group than the slow improvement group. Participants who started a biologic in the first month had more than five times greater odds of being in the rapid improvement group than in the slow improvement group (odds ratio [OR], 5.33; P = .017).
Those who started a biologic in the second month were also more likely to be in the rapid improvement group (OR, 2.67; P = .032). For those who began a biologic by the third month, the odds of improving rapidly were not statistically significant, though Ong noted that could have been because of the small sample size. There was also no significant difference between those who improved moderately vs slowly based on when a biologic was initiated.
It would be helpful to learn whether any of the patients in the rapid improvement group were able to stop medications or whether they all continued treatment during the 3 years of follow-up, Dr. Twilt said. “Does early treatment with biologics not only lead to early remission after initiation but also to the possibility of stopping treatment earlier and remaining in remission?” she asked.
The researchers also found that not all patients needed biologics to end up in the rapid improvement group. Among patients who never received any biologics during the 3-year period, 83% improved rapidly and 17% improved moderately. Yet the researchers identified no significant differences in demographics or clinical factors between patients who received biologics and those who did not.
“The fact that there is a group of patients in the rapid response group who never need a biologic is of great interest, as we always want to treat patients early with the medications they need, but we also want to avoid overtreating patients,” Dr. Twilt said. It’s important to find out what differentiates those patients and whether it is possible to predict which patients do not need biologics early on, she said.
Dr. Ong said the research team is working to develop machine learning methods to improve risk stratification in hopes of addressing that question.
Dr. Ong and Dr. Twilt reported no disclosures. The research was funded by CARRA and the Patient-Centered Outcomes Research Institute.
A version of this article appeared on Medscape.com .
CORRECTED April 16, 2024 // An earlier version of this article stated incorrect percentages of patients who never received any biologics during the study's 3-year period but improved rapidly or moderately.
Early initiation of biologics — within the first 2 months of symptom presentation — appears to have a significant impact on how rapidly patients with juvenile idiopathic arthritis (JIA) improve, according to findings presented at the annual scientific meeting of the Childhood Arthritis and Rheumatology Research Alliance.
“Our study provides evidence that early use of biologics can significantly alter the disease trajectory of patients with JIA,” Mei-Sing Ong, PhD, of Harvard Medical School, Boston, told attendees. At the same time, however, not all patients who improved rapidly during a 3-year follow-up period needed biologics, a finding that Ong said the researchers are continuing to investigate.
Marinka Twilt, MD, MScE, PhD, chair of CARRA’s JIA Research Committee and a pediatric rheumatologist and clinician scientist at Alberta Children’s Hospital in Calgary, Canada, was not involved in the research but said the continued sustained remission in patients who improved rapidly is very reassuring.
“We always wonder if initial response will be sustained or if patients tend to flare after the initial treatment,” Dr. Twilt told this news organization. “To see the sustained response up to 3 years is fantastic.” She added that it would be enlightening to see more information about patients who rapidly improved over 3 years, including whether they were still taking a [conventional disease-modifying antirheumatic drug (DMARD)] and/or biologic.
“A new diagnosis can be overwhelming for families, and this sometimes leads to step-up therapy to not overwhelm them more with information on new drugs,” Dr. Twilt said. “This study shows that an earlier start is beneficial, and this should be discussed with families early on so there is less delay in early treatment.”
Canada and many US states currently require 3 months of conventional DMARD treatment before patients can start a biologic, Dr. Twilt said, yet “this study shows the additive benefit of using a biologic within 2 months of starting a DMARD, which hopefully will lead to insurance companies adopting this threshold.”
The STOP-JIA study is a prospective observational study that compares the effectiveness of three different treatment plans for JIA. A Step-Up cohort of 257 patients received conventional antirheumatic monotherapy initially, with a biologic added at 3 months or later as needed. The Early Combination cohort of 100 patients received conventional antirheumatic therapy with a biologic from the start. The Biologic First cohort of 43 patients began taking a biologic as a first-line therapy.
In previously reported results of the study at 12 months’ follow-up, there was no significant difference between the Step-Up and Biologic First groups, but there were significant differences between the Step-Up and Early Combination groups. Significantly more patients in the Early Combination group (58.8%) than in the Step-Up group (42.8%) had inactive disease, based on the clinical Juvenile Arthritis Disease Activity Score 10 (cJADAS-10) (P = .03). Similarly, 81% of Early Combination patients achieved the American College of Rheumatology 70% improvement criteria, compared with 62% of the Step-Up patients (P = .01).
To learn whether the timing of starting a biologic influenced the disease trajectory over time, the researchers compared subgroups of patients with similar trajectories.
“Assessing treatment outcomes at a single point in time does not give us a complete picture of the effects of treatment on disease trajectory, which is an important outcome given that JIA is characterized by a relapsing-remitting course,” Dr. Ong told attendees.
Patients were sorted in the slow, moderate, or rapid improvement trajectories. In previously reported data at 12 months’ follow-up, patients’ odds of achieving rapid improvement were 3.6 times greater if they had started a biologic within 3 months.
This study compared patients’ trajectories over 3 years in the 259 patients (65% of the original cohort) who had at least one cJADAS-10 assessment in each year of follow-up. Most patients (66.8%) were in the rapid improvement class, with 25.9% in the moderate improvement class and 7.3% in the slow improvement class.
Patients in the rapid improvement group achieved inactive disease (cJADAS-10 of 2.5 or less) within 1 year and maintained inactive disease through the second and third years. The moderate and low improvement groups both had higher disease activity at baseline, but the moderate group continued to improve in years 2 and 3, with minimal disease by year 3, on the basis of the cJADAS-10 scores of 2.5-5. The slow group continued to experience moderate disease activity during years 2 and 3.
The findings also revealed that the earlier patients began a biologic, the more likely they were to be in the rapid improvement group than the slow improvement group. Participants who started a biologic in the first month had more than five times greater odds of being in the rapid improvement group than in the slow improvement group (odds ratio [OR], 5.33; P = .017).
Those who started a biologic in the second month were also more likely to be in the rapid improvement group (OR, 2.67; P = .032). For those who began a biologic by the third month, the odds of improving rapidly were not statistically significant, though Ong noted that could have been because of the small sample size. There was also no significant difference between those who improved moderately vs slowly based on when a biologic was initiated.
It would be helpful to learn whether any of the patients in the rapid improvement group were able to stop medications or whether they all continued treatment during the 3 years of follow-up, Dr. Twilt said. “Does early treatment with biologics not only lead to early remission after initiation but also to the possibility of stopping treatment earlier and remaining in remission?” she asked.
The researchers also found that not all patients needed biologics to end up in the rapid improvement group. Among patients who never received any biologics during the 3-year period, 83% improved rapidly and 17% improved moderately. Yet the researchers identified no significant differences in demographics or clinical factors between patients who received biologics and those who did not.
“The fact that there is a group of patients in the rapid response group who never need a biologic is of great interest, as we always want to treat patients early with the medications they need, but we also want to avoid overtreating patients,” Dr. Twilt said. It’s important to find out what differentiates those patients and whether it is possible to predict which patients do not need biologics early on, she said.
Dr. Ong said the research team is working to develop machine learning methods to improve risk stratification in hopes of addressing that question.
Dr. Ong and Dr. Twilt reported no disclosures. The research was funded by CARRA and the Patient-Centered Outcomes Research Institute.
A version of this article appeared on Medscape.com .
CORRECTED April 16, 2024 // An earlier version of this article stated incorrect percentages of patients who never received any biologics during the study's 3-year period but improved rapidly or moderately.
Early initiation of biologics — within the first 2 months of symptom presentation — appears to have a significant impact on how rapidly patients with juvenile idiopathic arthritis (JIA) improve, according to findings presented at the annual scientific meeting of the Childhood Arthritis and Rheumatology Research Alliance.
“Our study provides evidence that early use of biologics can significantly alter the disease trajectory of patients with JIA,” Mei-Sing Ong, PhD, of Harvard Medical School, Boston, told attendees. At the same time, however, not all patients who improved rapidly during a 3-year follow-up period needed biologics, a finding that Ong said the researchers are continuing to investigate.
Marinka Twilt, MD, MScE, PhD, chair of CARRA’s JIA Research Committee and a pediatric rheumatologist and clinician scientist at Alberta Children’s Hospital in Calgary, Canada, was not involved in the research but said the continued sustained remission in patients who improved rapidly is very reassuring.
“We always wonder if initial response will be sustained or if patients tend to flare after the initial treatment,” Dr. Twilt told this news organization. “To see the sustained response up to 3 years is fantastic.” She added that it would be enlightening to see more information about patients who rapidly improved over 3 years, including whether they were still taking a [conventional disease-modifying antirheumatic drug (DMARD)] and/or biologic.
“A new diagnosis can be overwhelming for families, and this sometimes leads to step-up therapy to not overwhelm them more with information on new drugs,” Dr. Twilt said. “This study shows that an earlier start is beneficial, and this should be discussed with families early on so there is less delay in early treatment.”
Canada and many US states currently require 3 months of conventional DMARD treatment before patients can start a biologic, Dr. Twilt said, yet “this study shows the additive benefit of using a biologic within 2 months of starting a DMARD, which hopefully will lead to insurance companies adopting this threshold.”
The STOP-JIA study is a prospective observational study that compares the effectiveness of three different treatment plans for JIA. A Step-Up cohort of 257 patients received conventional antirheumatic monotherapy initially, with a biologic added at 3 months or later as needed. The Early Combination cohort of 100 patients received conventional antirheumatic therapy with a biologic from the start. The Biologic First cohort of 43 patients began taking a biologic as a first-line therapy.
In previously reported results of the study at 12 months’ follow-up, there was no significant difference between the Step-Up and Biologic First groups, but there were significant differences between the Step-Up and Early Combination groups. Significantly more patients in the Early Combination group (58.8%) than in the Step-Up group (42.8%) had inactive disease, based on the clinical Juvenile Arthritis Disease Activity Score 10 (cJADAS-10) (P = .03). Similarly, 81% of Early Combination patients achieved the American College of Rheumatology 70% improvement criteria, compared with 62% of the Step-Up patients (P = .01).
To learn whether the timing of starting a biologic influenced the disease trajectory over time, the researchers compared subgroups of patients with similar trajectories.
“Assessing treatment outcomes at a single point in time does not give us a complete picture of the effects of treatment on disease trajectory, which is an important outcome given that JIA is characterized by a relapsing-remitting course,” Dr. Ong told attendees.
Patients were sorted in the slow, moderate, or rapid improvement trajectories. In previously reported data at 12 months’ follow-up, patients’ odds of achieving rapid improvement were 3.6 times greater if they had started a biologic within 3 months.
This study compared patients’ trajectories over 3 years in the 259 patients (65% of the original cohort) who had at least one cJADAS-10 assessment in each year of follow-up. Most patients (66.8%) were in the rapid improvement class, with 25.9% in the moderate improvement class and 7.3% in the slow improvement class.
Patients in the rapid improvement group achieved inactive disease (cJADAS-10 of 2.5 or less) within 1 year and maintained inactive disease through the second and third years. The moderate and low improvement groups both had higher disease activity at baseline, but the moderate group continued to improve in years 2 and 3, with minimal disease by year 3, on the basis of the cJADAS-10 scores of 2.5-5. The slow group continued to experience moderate disease activity during years 2 and 3.
The findings also revealed that the earlier patients began a biologic, the more likely they were to be in the rapid improvement group than the slow improvement group. Participants who started a biologic in the first month had more than five times greater odds of being in the rapid improvement group than in the slow improvement group (odds ratio [OR], 5.33; P = .017).
Those who started a biologic in the second month were also more likely to be in the rapid improvement group (OR, 2.67; P = .032). For those who began a biologic by the third month, the odds of improving rapidly were not statistically significant, though Ong noted that could have been because of the small sample size. There was also no significant difference between those who improved moderately vs slowly based on when a biologic was initiated.
It would be helpful to learn whether any of the patients in the rapid improvement group were able to stop medications or whether they all continued treatment during the 3 years of follow-up, Dr. Twilt said. “Does early treatment with biologics not only lead to early remission after initiation but also to the possibility of stopping treatment earlier and remaining in remission?” she asked.
The researchers also found that not all patients needed biologics to end up in the rapid improvement group. Among patients who never received any biologics during the 3-year period, 83% improved rapidly and 17% improved moderately. Yet the researchers identified no significant differences in demographics or clinical factors between patients who received biologics and those who did not.
“The fact that there is a group of patients in the rapid response group who never need a biologic is of great interest, as we always want to treat patients early with the medications they need, but we also want to avoid overtreating patients,” Dr. Twilt said. It’s important to find out what differentiates those patients and whether it is possible to predict which patients do not need biologics early on, she said.
Dr. Ong said the research team is working to develop machine learning methods to improve risk stratification in hopes of addressing that question.
Dr. Ong and Dr. Twilt reported no disclosures. The research was funded by CARRA and the Patient-Centered Outcomes Research Institute.
A version of this article appeared on Medscape.com .
FROM CARRA 2024
Active Surveillance for Cancer Doesn’t Increase Malpractice Risk
TOPLINE:
METHODOLOGY:
- Although practice guidelines from the National Comprehensive Cancer Network consider active surveillance an effective strategy for managing low-risk cancers, some physicians have been hesitant to incorporate it into their practice because of concerns about potential litigation.
- Researchers used Westlaw Edge and LexisNexis Advance databases to identify malpractice trends involving active surveillance related to thyroid, prostate, kidney, and or from 1990 to 2022.
- Data included unpublished cases, trial orders, jury verdicts, and administrative decisions.
- Researchers identified 201 malpractice cases across all low-risk cancers in the initial screening. Out of these, only five cases, all , involved active surveillance as the point of allegation.
TAKEAWAY:
- Out of the five prostate cancer cases, two involved incarcerated patients with Gleason 6 very-low-risk prostate adenocarcinoma that was managed with active surveillance by their urologists.
- In these two cases, the patients claimed that active surveillance violated their 8th Amendment right to be free from cruel or unusual punishment. In both cases, there was no metastasis or spread detected and the court determined active surveillance management was performed under national standards.
- The other three cases involved litigation claiming that active surveillance was not explicitly recommended as a treatment option for patients who all had very-low-risk prostate adenocarcinoma and had reported negligence from an intervention ( or cryoablation). However, all cases had documented informed consent for active surveillance.
- No relevant cases were found relating to active surveillance in any other type of cancer, whether in an initial diagnosis or recurrence.
IN PRACTICE:
“This data should bolster physicians’ confidence in recommending active surveillance for their patients when it is an appropriate option,” study coauthor Timothy Daskivich, MD, assistant professor of surgery at Cedars-Sinai Medical Center, Los Angeles, said in a statement . “Active surveillance maximizes quality of life and avoids unnecessary overtreatment, and it does not increase medicolegal liability to physicians, as detailed in the case dismissals identified in this study.”
SOURCE:
This study, led by Samuel Chang, JD, with Athene Law LLP, San Francisco, was recently published in Annals of Surgery.
LIMITATIONS:
The Westlaw and Lexis databases may not contain all cases or decisions issued by a state regulatory agency, like a medical board. Federal and state decisions from lower courts may not be published and available. Also, settlements outside of court or suits filed and not pursued were not included in the data.
DISCLOSURES:
The researchers did not provide any disclosures.
A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Although practice guidelines from the National Comprehensive Cancer Network consider active surveillance an effective strategy for managing low-risk cancers, some physicians have been hesitant to incorporate it into their practice because of concerns about potential litigation.
- Researchers used Westlaw Edge and LexisNexis Advance databases to identify malpractice trends involving active surveillance related to thyroid, prostate, kidney, and or from 1990 to 2022.
- Data included unpublished cases, trial orders, jury verdicts, and administrative decisions.
- Researchers identified 201 malpractice cases across all low-risk cancers in the initial screening. Out of these, only five cases, all , involved active surveillance as the point of allegation.
TAKEAWAY:
- Out of the five prostate cancer cases, two involved incarcerated patients with Gleason 6 very-low-risk prostate adenocarcinoma that was managed with active surveillance by their urologists.
- In these two cases, the patients claimed that active surveillance violated their 8th Amendment right to be free from cruel or unusual punishment. In both cases, there was no metastasis or spread detected and the court determined active surveillance management was performed under national standards.
- The other three cases involved litigation claiming that active surveillance was not explicitly recommended as a treatment option for patients who all had very-low-risk prostate adenocarcinoma and had reported negligence from an intervention ( or cryoablation). However, all cases had documented informed consent for active surveillance.
- No relevant cases were found relating to active surveillance in any other type of cancer, whether in an initial diagnosis or recurrence.
IN PRACTICE:
“This data should bolster physicians’ confidence in recommending active surveillance for their patients when it is an appropriate option,” study coauthor Timothy Daskivich, MD, assistant professor of surgery at Cedars-Sinai Medical Center, Los Angeles, said in a statement . “Active surveillance maximizes quality of life and avoids unnecessary overtreatment, and it does not increase medicolegal liability to physicians, as detailed in the case dismissals identified in this study.”
SOURCE:
This study, led by Samuel Chang, JD, with Athene Law LLP, San Francisco, was recently published in Annals of Surgery.
LIMITATIONS:
The Westlaw and Lexis databases may not contain all cases or decisions issued by a state regulatory agency, like a medical board. Federal and state decisions from lower courts may not be published and available. Also, settlements outside of court or suits filed and not pursued were not included in the data.
DISCLOSURES:
The researchers did not provide any disclosures.
A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Although practice guidelines from the National Comprehensive Cancer Network consider active surveillance an effective strategy for managing low-risk cancers, some physicians have been hesitant to incorporate it into their practice because of concerns about potential litigation.
- Researchers used Westlaw Edge and LexisNexis Advance databases to identify malpractice trends involving active surveillance related to thyroid, prostate, kidney, and or from 1990 to 2022.
- Data included unpublished cases, trial orders, jury verdicts, and administrative decisions.
- Researchers identified 201 malpractice cases across all low-risk cancers in the initial screening. Out of these, only five cases, all , involved active surveillance as the point of allegation.
TAKEAWAY:
- Out of the five prostate cancer cases, two involved incarcerated patients with Gleason 6 very-low-risk prostate adenocarcinoma that was managed with active surveillance by their urologists.
- In these two cases, the patients claimed that active surveillance violated their 8th Amendment right to be free from cruel or unusual punishment. In both cases, there was no metastasis or spread detected and the court determined active surveillance management was performed under national standards.
- The other three cases involved litigation claiming that active surveillance was not explicitly recommended as a treatment option for patients who all had very-low-risk prostate adenocarcinoma and had reported negligence from an intervention ( or cryoablation). However, all cases had documented informed consent for active surveillance.
- No relevant cases were found relating to active surveillance in any other type of cancer, whether in an initial diagnosis or recurrence.
IN PRACTICE:
“This data should bolster physicians’ confidence in recommending active surveillance for their patients when it is an appropriate option,” study coauthor Timothy Daskivich, MD, assistant professor of surgery at Cedars-Sinai Medical Center, Los Angeles, said in a statement . “Active surveillance maximizes quality of life and avoids unnecessary overtreatment, and it does not increase medicolegal liability to physicians, as detailed in the case dismissals identified in this study.”
SOURCE:
This study, led by Samuel Chang, JD, with Athene Law LLP, San Francisco, was recently published in Annals of Surgery.
LIMITATIONS:
The Westlaw and Lexis databases may not contain all cases or decisions issued by a state regulatory agency, like a medical board. Federal and state decisions from lower courts may not be published and available. Also, settlements outside of court or suits filed and not pursued were not included in the data.
DISCLOSURES:
The researchers did not provide any disclosures.
A version of this article appeared on Medscape.com.